
Experience the future
of Pharma Manufacturing.
Plan, execute, and optimize every batch. Deliver On Time In Full with AI-powered pharma manufacturing & compliance intelligence.























Integrated Platform to Plan, Execute, and Optimize Every Batch—On Time, In Full.
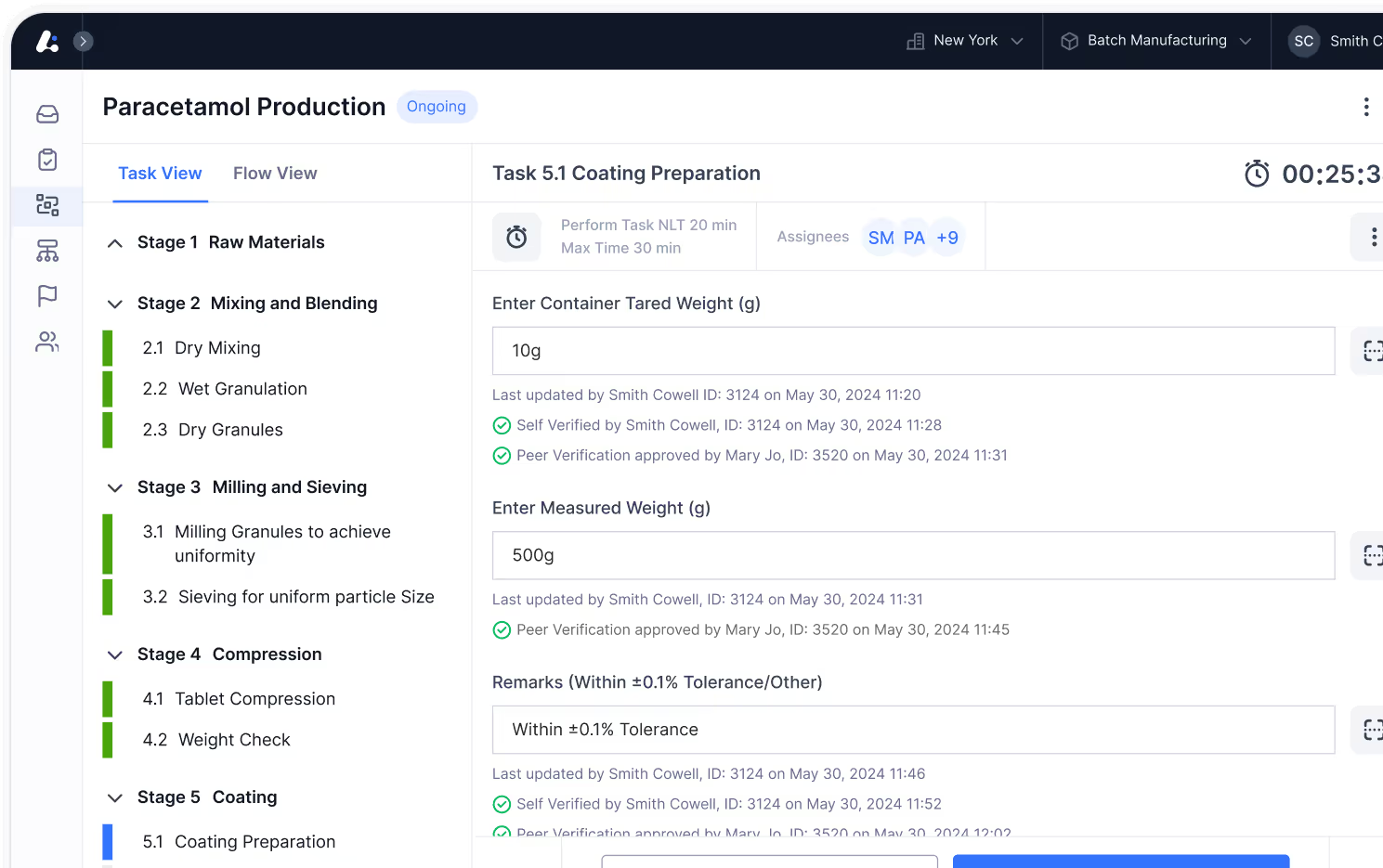
Track every batch from planning to release—identify constraints, detect bottlenecks, and take proactive actions to eliminate delays.






Manufacturing
Execution System
Optimize every step of production, from material dispensing to final batch release.


Quality
Management System
Ensure seamless regulatory compliance and quality excellence with AI-driven quality solutions.

Laboratory
Execution System
Transform lab operations with AI-powered automation.
Unlock Tangible Benefits with Leucine
Track every batch from planning to release—identify constraints, detect bottlenecks, and take proactive actions to eliminate delays.














.png)
.png)
.png)


.png)
.png)
.png)



















