FDA Observations CAPA

1. Failure to Review Discrepancies Leads to Repeated Production Issues
Possible Root Cause:
Inadequate investigations, ineffective CAPA system, insufficient filling line monitoring, weak stability testing.
Corrective Action:
Revise investigation procedures, enhance CAPA system, implement stringent filling line controls, develop robust stability testing.
Preventive Action:
Retrain staff, install monitoring systems, conduct risk assessments, establish routine review meetings.
Reference FDA 483:
Company Name: Eugia Pharma Specialities Limited
Issue Date: 02-Feb-24
Inspection Dates: 22 Jan 2024 to 02 Feb 2024
Investigators: Justin A Boyd, Eileen A Liu, Anastasia M Shields
2. Systemic Weaknesses in Handling Discrepancies Risk Product Quality
Possible Root Cause:
Weak investigation process, inadequate lot connections, delayed completions.
Corrective Action:
Revamp investigation processes, extend analyses to impacted batches, implement training for investigative rigor.
Preventive Action:
Introduce tracking for investigation timelines, establish a continuous improvement program to identify trends and mitigate recurrences.
Reference FDA 483:
Company Name: Stokes Healthcare Inc. dba Epicur Pharma
Issue Date: 25-Oct-23
Inspection Dates: 26 Sep 2023 to 25 Oct 2023
Investigators: Christina K Theodorou, Yoriann Rodriguez
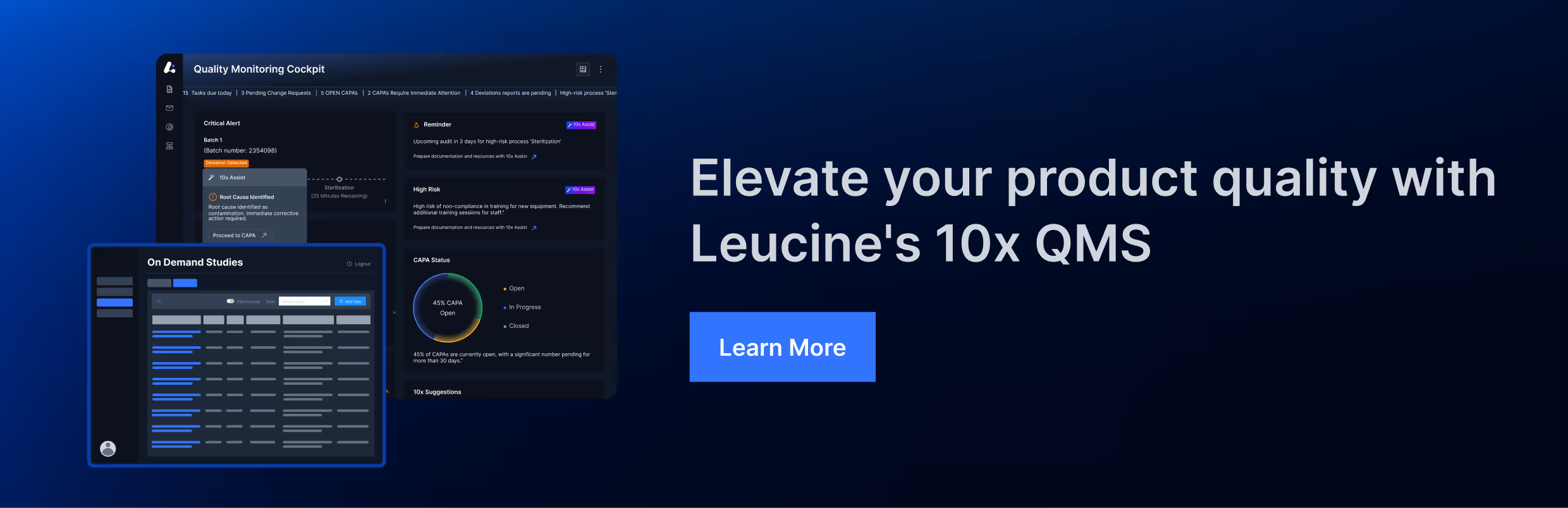
3. SOPs Allow Bypassing Thorough Investigations, Risking Sterility Assurance
Possible Root Cause:
Lax procedures, inadequate investigations, insufficient sterility controls, systemic aseptic issues.
Corrective Action:
Revise SOPs, train staff, enhance environmental monitoring, evaluate sporicidal disinfectants' effectiveness.
Preventive Action:
Routine CAPA reassessment, increased quality oversight, robust validation, comprehensive environmental monitoring.
Reference FDA 483:
Company Name: SCA Pharmaceuticals, LLC
Issue Date: 20-Oct-23
Inspection Dates: 18 Sep 2023 to 20 Oct 2023
Investigators: Jonah S Ufferfilge, John P Mistell
4. Deficient Investigation Processes Risk Recurring Quality Issues
Possible Root Cause:
Poorly defined procedures, inadequate training, weak documentation, limited investigation scope.
Corrective Action:
Develop comprehensive SOPs, conduct training, enhance record-keeping, expand investigation scope.
Preventive Action:
Regular training programs, periodic senior reviews, digital tracking, risk-based investigation approach.
Reference FDA 483:
Company Name: Sichuan Deebio Pharmaceutical Co. Ltd.
Issue Date: 08-Sep-23
Inspection Dates: 04 Sep 2023 to 08 Sep 2023
Investigators: Arsen Karapetyan, Anders W Evenson
5. High Mold Recoveries Reveal Inadequate Environmental Controls
Possible Root Cause:
Inadequate monitoring, lax action limits, ineffective CAPA measures.
Corrective Action:
Enforce stricter monitoring, implement targeted cleaning, thoroughly investigate microbial excursions.
Preventive Action:
Review and improve monitoring strategies, enhance staff training, regularly update CAPA procedures.
Reference FDA 483:
Company Name: Alvotech Hf
Issue Date: 22-Mar-22
Inspection Dates: 10 Mar 2022 to 17 Mar 2022
Investigators: Madushini Dharmasena, PhD, Senior Pharmacist
6. Management Awareness of Safety Issues Reveals Quality Failures
Possible Root Cause:
Ineffective oversight, poor risk management, weak CAPA processes, inadequate communication.
Corrective Action:
Review management and CAPA processes, enhance training, develop robust risk management, reevaluate product designs.
Preventive Action:
Regular management reviews, routine CAPA and risk audits, improved training programs, better communication channels.
Reference FDA 483:
Company Name: Philips Respironics, Inc.
Issue Date: 09-Nov-21
Inspection Dates: 26 Aug 2021 to 09 Nov 2021
Investigators: Katelyn A Staub-Zamperini

7. Systemic Issues in Quality Control Require Comprehensive Overhaul
Possible Root Cause:
Lack of procedures, ineffective investigations, delayed reporting, insufficient documentation, weak CAPA implementation.
Corrective Action:
Implement comprehensive written procedures, conduct thorough investigations, update reporting systems, review supplier qualifications, enhance CAPA.
Preventive Action:
Regular training, periodic quality reviews, risk management approach, routine audits of processes.
Reference FDA 483:
Company Name: Empower Clinic Services LLC dba Empower Pharmacy
Issue Date: 05-Aug-22
Inspection Dates: 18 Jul 2022 to 05 Aug 2022
Investigators: Margaret M Annes, Suzanne N Vallez

























.png)
