FDA 483 Response: What Happens After an FDA Inspection? From 483 to Final Enforcement Action
Learn how to craft a timely, effective FDA 483 response within 15 business days. Ensure compliance, avoid escalation, and protect product quality.

An FDA inspection is just the beginning for companies, such as medical device and IVD manufacturers. This article addresses what happens after FDA inspections, focusing on the steps companies should take once the inspector leaves. The importance of compliance and timely action after an inspection cannot be overstated, as prioritizing adherence to FDA regulations is essential for preventing issues and fostering a culture of accountability within the organization. Whether you received a clean report or were issued a Form FDA 483, how you manage the post-inspection period is critical.
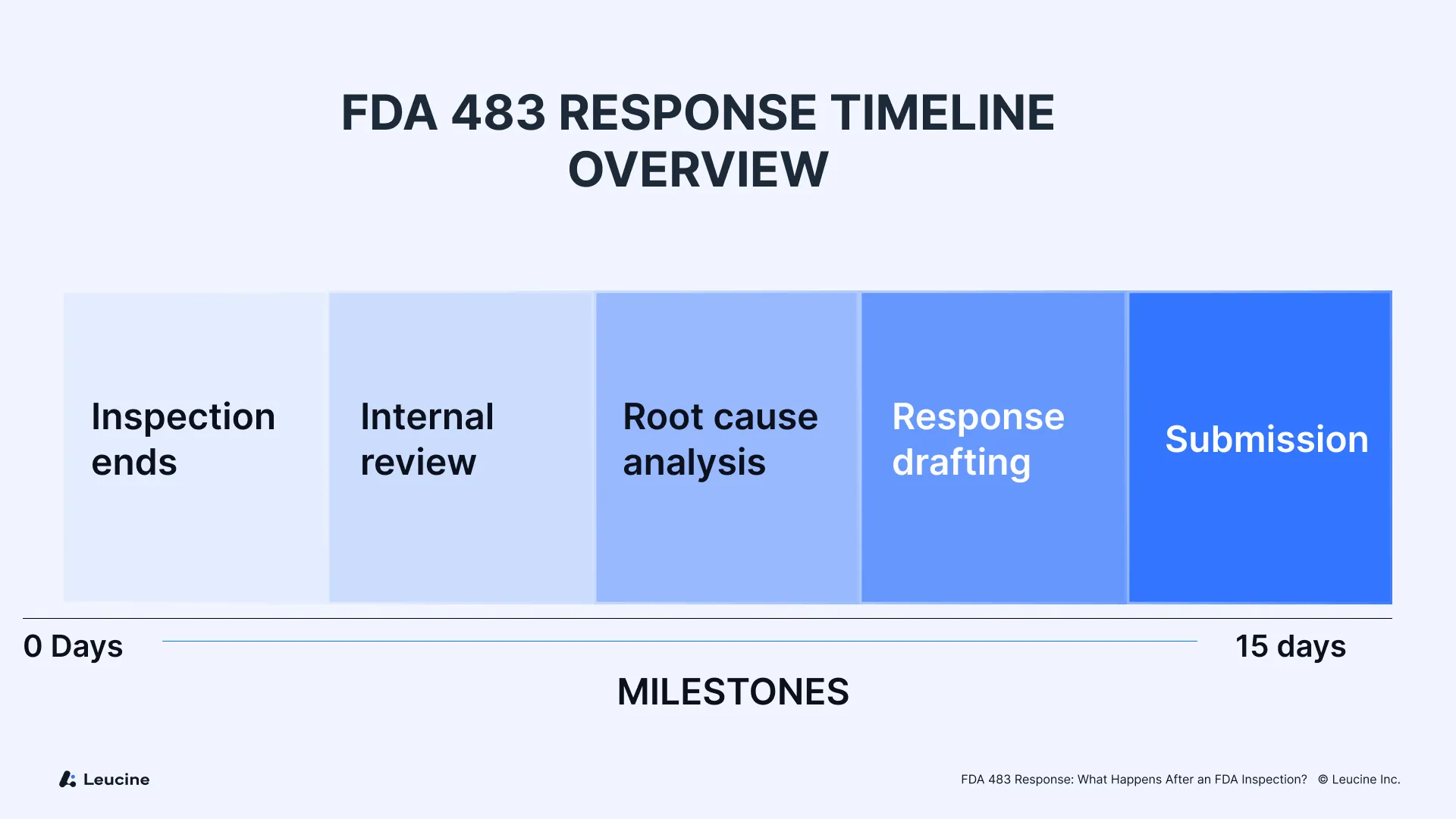
A thoughtful, timely FDA 483 response is essential for responding to regulatory observations and can mitigate risks, prevent warning letters, and protect your company from further regulatory actions. Companies must submit an initial response within 15 business days, outlining corrective actions and setting expectations for follow-up updates. Responding promptly and thoroughly to FDA 483 observations demonstrates your understanding of FDA regulations, observed conditions, and commitment to regulatory compliance.
Step 1: Internal Debrief & Document Review
Immediately after the inspection concludes:
- Conduct an internal debrief with the inspection response team, including a discussion of the inspection process and the findings of FDA investigators.
- Capture all verbal comments made by FDA inspectors.
- Review the Form 483 observations line-by-line with SMEs.
- Identify documentation requested, gaps observed, and verbal concerns.
- Discuss any points that were discussed during the inspection with the team to ensure clarity and alignment.
Some observations are based on the investigator's judgment and may reflect subjective assessments.
This initial analysis forms the backbone of your FDA 483 response and determines your corrective strategy. The team should review the context of the inspections and the judgment used by FDA investigators. Be prepared to provide objective evidence and address inspectional observations cited by the FDA investigator.
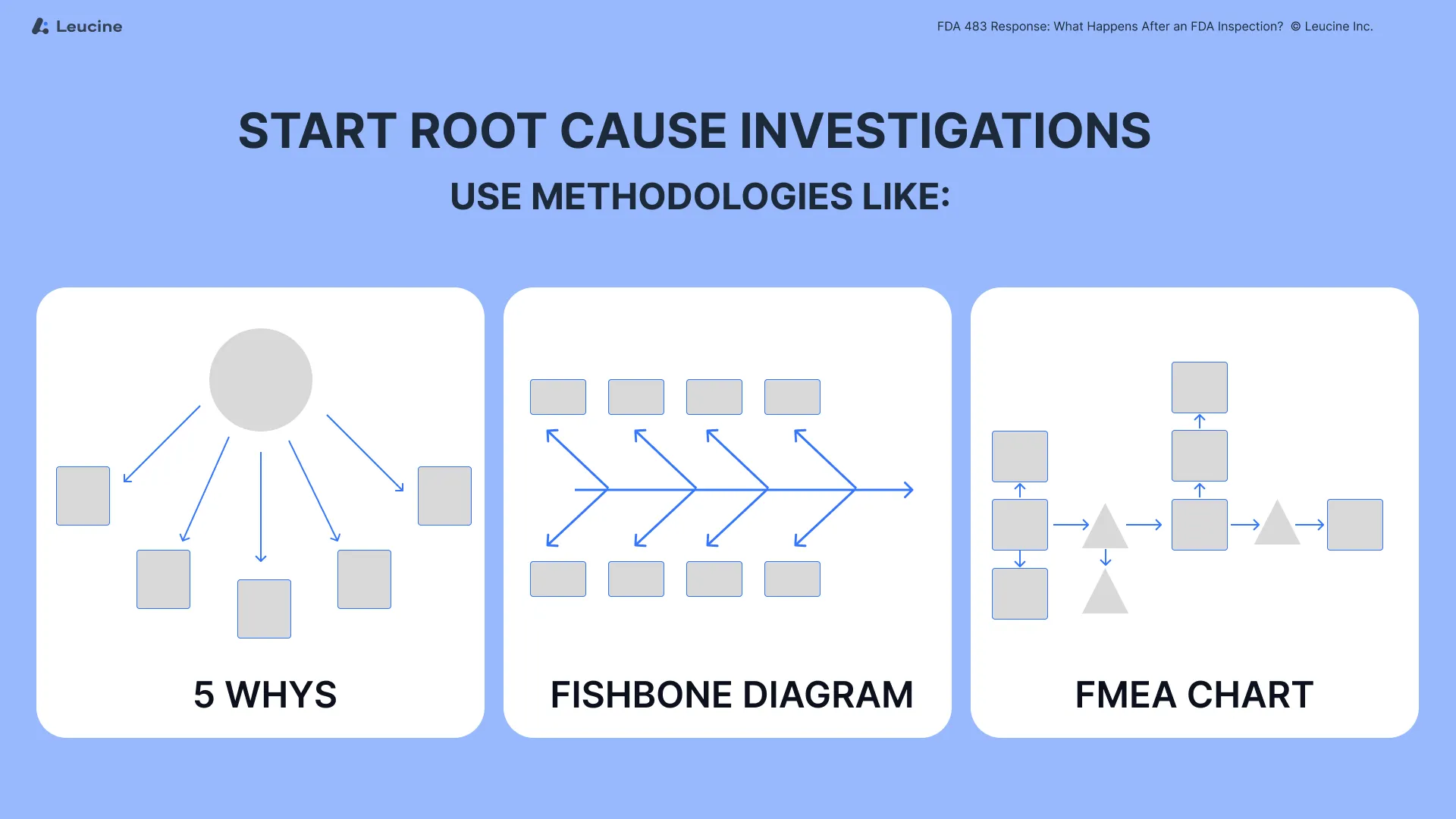
Step 2: Start Root Cause Investigations
Initiate root cause analysis (RCA) for every observation cited, focusing on the process of root cause investigation and correction. Avoid surface-level answers. Use methodologies like:
- 5 Whys
- Fishbone diagrams
- Failure Mode and Effects Analysis (FMEA)
These tools help ensure that corrections are properly implemented and that the process addresses the underlying issues.
Proper RCA strengthens your credibility and supports long-term corrective and preventive actions (CAPA). This approach helps identify systemic issues and enables you to implement effective corrective actions. Such actions should be systemic, not just immediate fixes, to prevent recurrence and ensure ongoing compliance.
Use FDA Tracker to benchmark your CAPAs against real 483 responses and industry best practices.
It is essential to ensure that all corrections are correct, thoroughly documented, and fully implemented to demonstrate compliance and address deficiencies effectively.
Step 3: Drafting the FDA 483 Response
The FDA expects a timely response within 15 business days from the end of the inspection. When writing your FDA 483 response, it is important to craft a clear and well-structured document that effectively communicates your actions and compliance.
Your FDA 483 response should include:
- Acknowledgment of each observation, providing context to clarify the background and significance
- Root cause summary
- Immediate containment actions taken, ensuring all actions are documented
- CAPA plans with due dates
- Supporting evidence where available
All responses should be written clearly and supported by documented evidence to demonstrate compliance and address each observation effectively.
Responses should be professional, factual, and structured. Address each point directly—don’t generalize. Describe your standard operating procedures and planned actions clearly. It’s crucial to write responses that are well-supported, provide sufficient context for the FDA to understand the actions taken, comply with FDA form expectations, and demonstrate a firm grasp of regulatory compliance.
Step 4: Assemble a Response Package
When assembling your response package for the FDA, ensure the formal issuance of your submission is clear and timely to address the agency’s expectations.
To strengthen your submission, include:
- Cover letter with an executive summary
- Tabulated list of each observation and response
- Reference documents: SOPs, training logs, change controls
- Timeline for completion of CAPAs
- Interim measures for risk mitigation
- An example of a well-documented response to illustrate best practices
Use version-controlled documentation and ensure all actions, evidence, and responses are thoroughly documented. This helps the agency verify your corrective action plan and the integrity of your quality systems.
Not sure what a strong response looks like? FDA Tracker shows real FDA 483 responses with outcomes. Explore examples.

Step 5: Monitor Follow-Up Actions
After your submission:
- Monitor CAPA implementation status internally.
- Track response timelines using a dashboard.
- Update internal stakeholders regularly.
- Prepare for a possible follow-up inspection or remote regulatory review.
- Ensure all violations are promptly addressed to comply with the Food, Drug, and Cosmetic (FD&C) Act.
A weak or incomplete response can lead to:
- Warning Letters (issued if violations are not addressed)
- Import alerts
- Consent decrees
The establishment inspection report, analysis tools, and objective evidence will contribute to the final agency determination. These insights help determine whether your practices, processes, and procedures meet expectations or reflect potential violations under the FD&C Act. All issues must be addressed to avoid further regulatory action, such as a warning letter, especially in the context of food drug compliance.
How FDA Tracker Helps You Respond Better
FDA Tracker is your intelligence partner in post-inspection success. It helps:
- Analyze FDA form 483 observations
- Understand FDA inspector focus areas
- Benchmark CAPA timelines and effectiveness
- Track enforcement patterns from inspection to final action
- Provide support for drafting well-prepared responses, including objective evidence and documentation
FDA Tracker incorporates insights from trained professionals, ensuring you are well prepared for inspections and responses. It guides you in creating an effective FDA 483 response by highlighting best practices and key steps for communication with the FDA.
By studying historical data and current trends, you can make your FDA 483 response stronger, smarter, and on time. It enables you to anticipate objectionable conditions, prepare with background information, and implement measures to maintain compliance.
Start using FDA Tracker for free to turn your next 483 into a compliance win.


















