FDA Form 483: Meaning, Impact & How to Stay Ahead
A Form 483 signals FDA concerns that can trigger warning letters. Learn what it means, how to respond, and how to prevent it before it’s too late.

When an FDA inspection concludes, the silence is often broken by a document that sets the tone for what comes next: the FDA Form 483.
Issued under the authority of the Food Drug and Cosmetic Act, this form signals that the FDA inspector has identified inspectional observations that may constitute violations of relevant laws or regulations. This blog post explains the fda 483 meaning, how to interpret it, what happens after you receive one, and how you can stay one step ahead using FDA Tracker.
What Is a FDA Form 483?
An FDA Form 483, also referred to simply as form 483, is a formal notification issued by an FDA investigator when practices observed during an inspection appear to constitute violations of the Federal Food, Drug, and Cosmetic Act or FDA regulations.
While not an enforcement action itself, a Form 483 provides written inspection observations that the fda expects a company to address through an effective response—often including a detailed corrective action plan.
It typically includes:
- Detailed observed conditions at the site
- Citations related to standard operating procedures, written procedures, or lack thereof
- Evidence suggesting potential violations of regulations
- The investigator’s judgment on whether these issues may render injurious the drug or medical device manufactured
📌 Bottom line: It’s your chance to respond before a final agency determination or warning letter is issued.
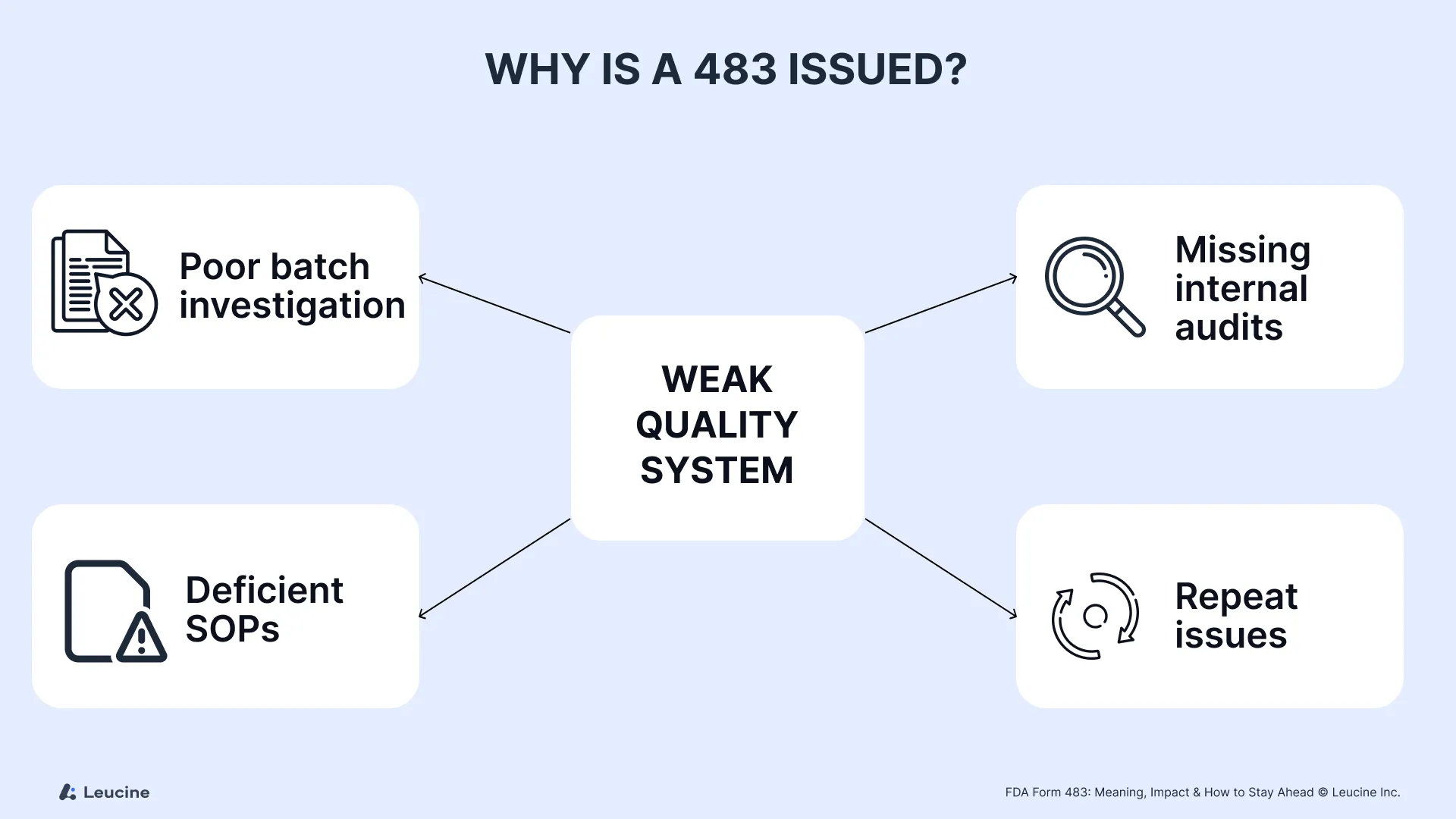
Why Is a 483 Issued?
A Form 483 is issued when an FDA inspector identifies gaps during an inspection that signal weaknesses in your quality system, documentation, or GMP controls. These observations often relate to written procedures, investigation failures, or systemic non-compliance.
Common causes include:
- Poor investigations of batch failures or OOS results
- Absence of internal audits to verify ongoing compliance
- Incomplete inspection reports or establishment inspection report (EIR) follow-up
- Deficient corrective actions or standard operating procedures
- Repeat observations from a prior inspection
Even global foreign manufacturers and companies with sophisticated supply chains are not exempt from the type of regulatory scrutiny Form 483 invites.
FDA 483 Meaning vs. Warning Letter
A Form 483 isn’t the final say—it’s the formal notification that opens the window for written response and remediation. A warning letter, however, signals that the agency has reviewed your response and found it inadequate.
A well-structured, thorough written response is your best defense.
📘 Learn what turns a 483 into a warning letter
What Happens After You Receive a Form 483?
Once a 483 FDA form is handed over, the clock starts ticking. Companies have just 15 working days to respond.
Your response should:
- Acknowledge each finding
- Provide evidence of containment and corrective actions
- Outline a corrective action plan tied to root cause
- Attach supporting documentation and assign responsibility
- Clearly show how your facility now prioritizes compliance
Failing to respond may lead to a warning letter, import alert, or even product seizure—particularly if the issues are severe, repeated, or related to drug safety, medical device integrity, or patient harm.

What Does a 483 Look Like?
A typical 483 document includes:
- Your company name and inspection date
- A list of numbered observations
- Descriptions of objectionable conditions or practices
- The investigator's judgment supported by direct inspectional observations
Under the Freedom of Information Act, these forms are often publicly posted on the FDA’s website, where they are viewed by even millions in the industry.
Download & Analyze and a real-world Form 483
Common Systems Flagged in 483s
The FDA uses a Six-System Inspection Model, where each system is evaluated independently. Most FDA 483 observations relate to gaps in the following areas:
- Quality System – e.g., failure to manage CAPA or incomplete SOPs
- Production System – errors in processes, line clearance, or cleaning
- Laboratory Controls – e.g., improper OOS handling or missing data
- Facilities & Equipment – HVAC, water systems, pest control, etc.
- Materials System – supply chain weaknesses or vendor qualification
- Packaging & Labeling System – mislabeling or reconciliation failures

Explore the most cited systems and related insights
How FDA Tracker Helps Prevent Form 483s
FDA Tracker empowers your team with data from thousands of fda form 483 records to detect risks and adjust before your next inspection.
With FDA Tracker, you can:
- Search for 483s by company, system, drug class, or keywords
- Understand how often certain practices are flagged by specific FDA investigators
- Monitor repeat observations and plan corrective actions proactively
- Conduct internal audits aligned with real inspection reports
- Stay alert to trends that affect your facility, region, or supply chain
Signup for Free on FDA Tracker
What Makes FDA Tracker Unique?
- 📊 Smart clustering of observations by system and language
- 🔍 Visibility into publicly posted enforcement history
- 🧠 Tools to improve corrective action plan quality
- 📥 Custom alerts for new 483s and fda warning letters
- 🧾 Benchmarking to show how your site compares against peers
💡 With FDA Tracker, you don’t wait for the agency to point out problems—you determine them first.
Final Thoughts: Form 483 Is a Wake-Up Call—Not a Death Sentence
If you've received a 483, it's not the end—it's a starting point. It’s your chance to prove that your quality system is built not only to correct but to continuously improve.
More importantly, if you haven’t received one yet, it’s your opportunity to address weaknesses now, build robust processes, and create a culture that truly prioritizes compliance.




















