What Triggers an FDA Audit: Understanding For-Cause Inspections and How to Respond
Learn why FDA audits happen and how to respond fast. Stay compliant, ensure data integrity, and build inspection readiness with real-time insights.

An FDA audit can occur at any time, but when it’s a for-cause inspection, it’s often the result of specific circumstances such as complaints, audit failures, or adverse events that raise serious concerns flagged by the agency.
These targeted inspections aim to investigate specific issues such as product complaints, adverse event reports, whistleblower tips, or prior inspection findings. Understanding what triggers them—and how to respond—can be the difference between a manageable outcome and a formal enforcement action.
Want to know how your peers are being audited? Use FDA Tracker to review real Form 483s and inspector trends
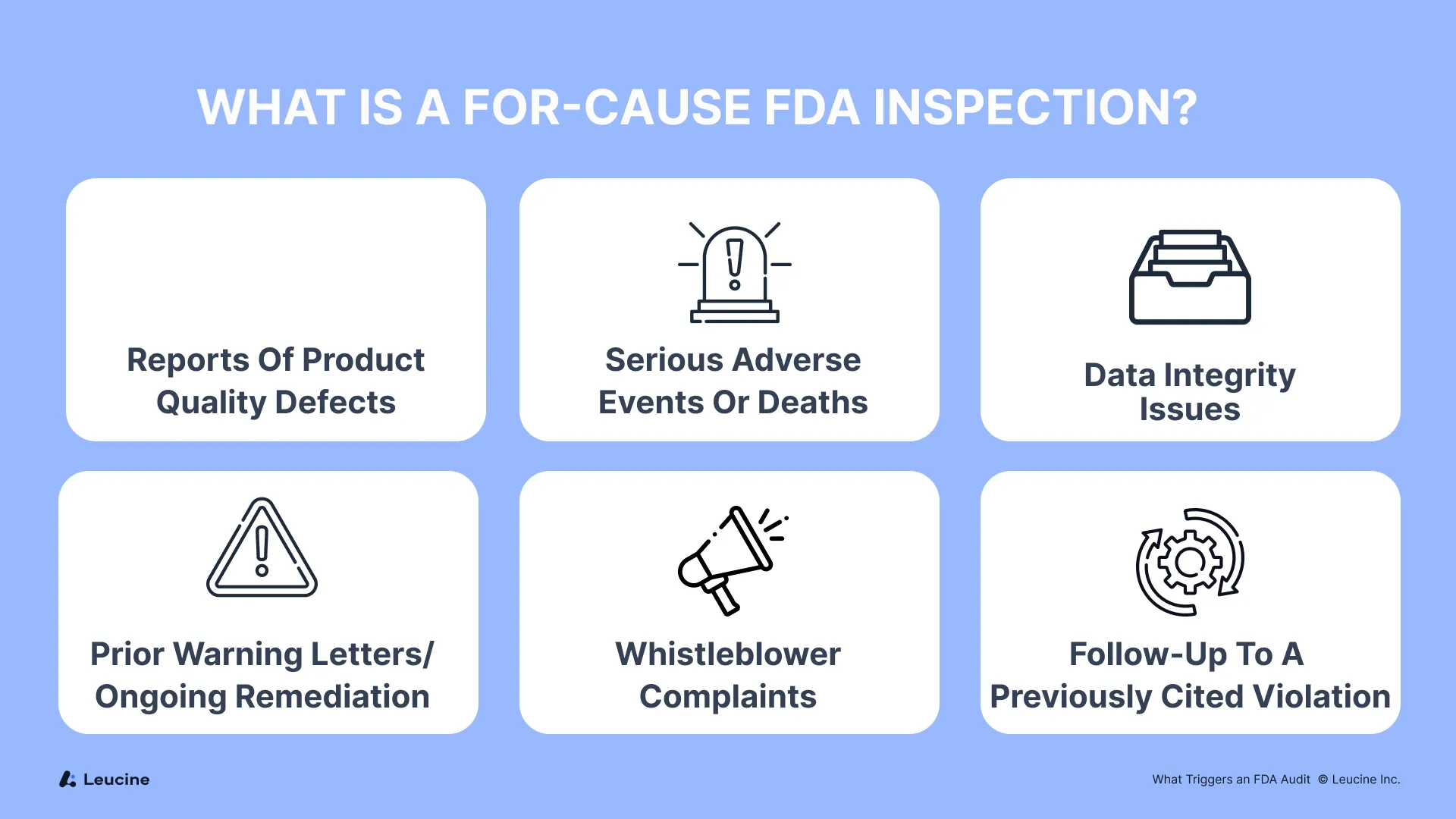
What Is a For-Cause FDA Inspection?
A for-cause inspection is a focused FDA audit prompted by a specific concern, often involving:
- Reports of product quality defects
- Serious adverse events or deaths
- Data integrity issues
- Prior warning letters or ongoing remediation
- Whistleblower complaints
- Follow-up to a previously cited violation
Unlike routine surveillance inspections, these are not scheduled. The FDA often arrives unannounced, expecting immediate access to relevant records and personnel. FDA inspectors conduct inspections to assess compliance with regulatory standards and determine if manufacturers are maintaining a robust quality system that ensures product safety.
The scope of a for-cause inspection may include reviewing the facility, quality system, and specific processes related to the identified concern. The FDA may focus on how well the facility is maintained and whether the quality system is properly documented and accessible during these inspections, which are conducted to ensure effective oversight and compliance.
Track trends across thousands of FDA audits to stay one step ahead. FDA Tracker shows patterns across facilities, products, and subsystems.
Common Triggers for a For-Cause FDA Audit
Proactively monitoring for potential issues is crucial to prevent for-cause inspections and ensure ongoing compliance.
- Adverse Events and Product Complaints
Unexpected side effects or failures reported through MedWatch or other pharmacovigilance systems can trigger inspections as the FDA seeks to identify potential issues. - Data Integrity Concerns
Missing or manipulated data in submissions, audit trails, or discrepancies in batch records often result in regulatory scrutiny to ensure all problems are properly identified and addressed. - Whistleblower Reports
Anonymous tips about GMP violations, safety risks, or non-compliance with procedures can lead to investigations focused on identifying and addressing potential issues. - Follow-Up to Previous Inspections
If a company received a Form 483 or warning letter, the FDA issues these notices to identify compliance deficiencies that must be addressed. The FDA might return to verify corrective actions and CAPA effectiveness. - Recalls or Field Alerts
Recalls due to safety, efficacy, or contamination issues often indicate quality problems that need to be identified and resolved.
How to Prepare for a For-Cause Inspection
1. Implement a Culture of Readiness
Preparing for FDA inspections means being ready at any time, even with little or no notice. All aspects of operations should be regularly reviewed to ensure compliance with FDA regulations and other regulations. Staff should demonstrate expertise in their roles during inspections, and every aspect of the facility and process should reflect best practices. Follow official guidelines and maintain up-to-date procedures to ensure ongoing readiness. Regularly review documentation trails and ensure all documents are organized for quick retrieval.
2. Use Mock Inspections
Simulate for-cause scenarios to assess how well your inspection team responds under pressure. Mock inspections provide support and oversight to the inspection team, helping to identify gaps and reinforce compliance with guidelines and regulations.
3. Audit Your CAPA System
Make sure corrective and preventive actions are documented, timely, and verifiably effective. The CAPA system should include validation of corrective actions and be reviewed regularly. Review complaint management, root cause analysis procedures, and processes—including manufacturing processes—for compliance with regulations and best practices.

4. Have a Response Protocol in Place
Identify your inspection coordinator, SME spokespersons, and documentation handlers. The response protocol should include clear roles for submitting documents and addressing inspector requests. It is important to be able to quickly find and provide reviewed documents during the inspection to demonstrate compliance and organization.
5. Review Past Inspection Data
Analyze past 483s, warning letters, and inspector behavior to anticipate what might be asked. Reviewing past inspection data helps manufacturers understand how authorities determine the scope of future inspections and prepare accordingly.
- Ensuring data integrity, reliability, and compliance with FDA regulations and other regulations is critical, especially in clinical trials, food, and manufacturing environments. Maintaining compliance supports continued market access and demonstrates oversight to regulatory authorities.
FDA Tracker gives you immediate access to historical inspection data to guide your preparation. Try it here.
Responding to a For-Cause FDA Inspection
1. Stay Calm and Follow Protocol
Activate your inspection response team. Escort the FDA inspector and begin tracking every document request and verbal question. Ensure that all requested documents are tracked and clearly marked as reviewed. Have your war room and back room communication ready.
2. Be Transparent but Precise
Only answer what is asked. Avoid speculation. Have your SMEs use the CLEAR method—Clarify, Listen, Express, Avoid speculation, and Remain factual. Ensure that all questions and concerns raised by the inspector are clearly addressed by the SME.
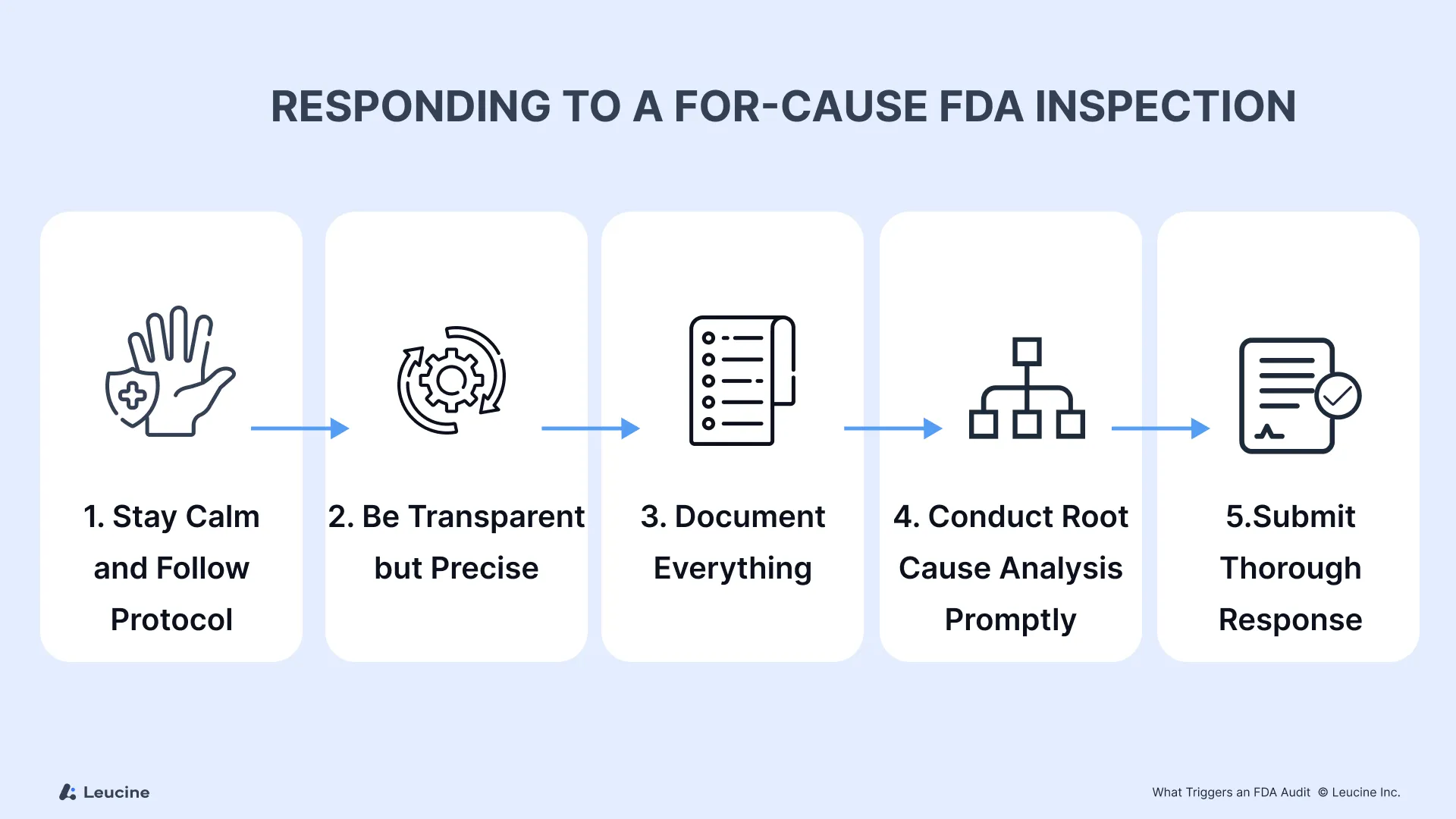
3. Document Everything
Maintain a backroom log of all requests, responses, and observations. This record will help during the post-inspection response phase and ensure traceability of confidential information and submitted data.
4. Conduct Root Cause Analysis Promptly
If issues are identified during the inspection, launch a formal root cause investigation while the inspection is ongoing. Provide initial containment steps and initiate corrective actions.
5. Prepare a Timely and Thorough Response
You have 15 business days for submitting your response to a Form 483. Outline each observation, include CAPA plans, verify effectiveness, and show systemic review of records and operations.
Use FDA Tracker to see how similar companies responded to 483s. Explore real-world examples.
How FDA Tracker Helps You Stay Audit-Ready
FDA Tracker is a real-time inspection intelligence platform that empowers quality and compliance teams with support for compliance initiatives, including:
- Access to the latest Form 483s and warning letters
- Investigator behavior profiles and enforcement patterns
- Insights on trending subsystems and audit focus areas
- Risk signals from across the industry to prepare proactively
By analyzing thousands of FDA inspections and audit findings, FDA Tracker helps you:
- Benchmark your inspection history against peers
- Understand what triggered audits across facilities and specific processes
- Understand the scope of inspections and audits to ensure thorough preparedness
- Train teams using actual FDA observations
- Plan preventive actions based on real trends
Whether it’s a medical device inspection, clinical trial monitoring, or routine surveillance, FDA Tracker enhances regulatory oversight and supports continuous improvement by providing the regulatory insight needed to maintain data integrity, ensure compliance, and safeguard patient safety.
Start using FDA Tracker for free to improve audit outcomes and build a smarter inspection readiness strategy.


















