What Is an FDA Warning Letter?
FDA warning letters signal serious compliance failures. Learn what they mean, what triggers them, and how to respond before it leads to regulatory action.

In the regulated industry of pharmaceuticals and life sciences, receiving an FDA warning letter is a moment of reckoning. These warning letters serve as the agency’s principal means of alerting companies about significant violations of federal regulations, and they carry serious regulatory significance.
Let’s break down what a warning letter really means, what triggers it, and the types of letters issued across various sectors of the FDA-regulated space.
What Is an FDA Warning Letter?
An FDA warning letter is an official correspondence from the agency to a letter recipient, notifying them of violations of the Federal Food, Drug, and Cosmetic Act (FDCA), tobacco control act, or other applicable regulations. These letters are not informal notices—they represent a formal enforcement action after an evaluation of your practices and written response to previous findings.
In most cases, the warning letter identifies conditions that must be adequately corrected within a short timeline, often 15 working days. These letters may be sent after a Form 483, when prompt corrective action is not taken, or when issues discussed have broader regulatory status implications.
Why Are FDA Warning Letters Issued?
The FDA issues warning letters when it has reason to believe a firm is in violation of the law and the problem warrants more than prior notice or untitled letters. These are not limited to drug manufacturing—they also apply to medical devices, tobacco products, and online prescription drugs.
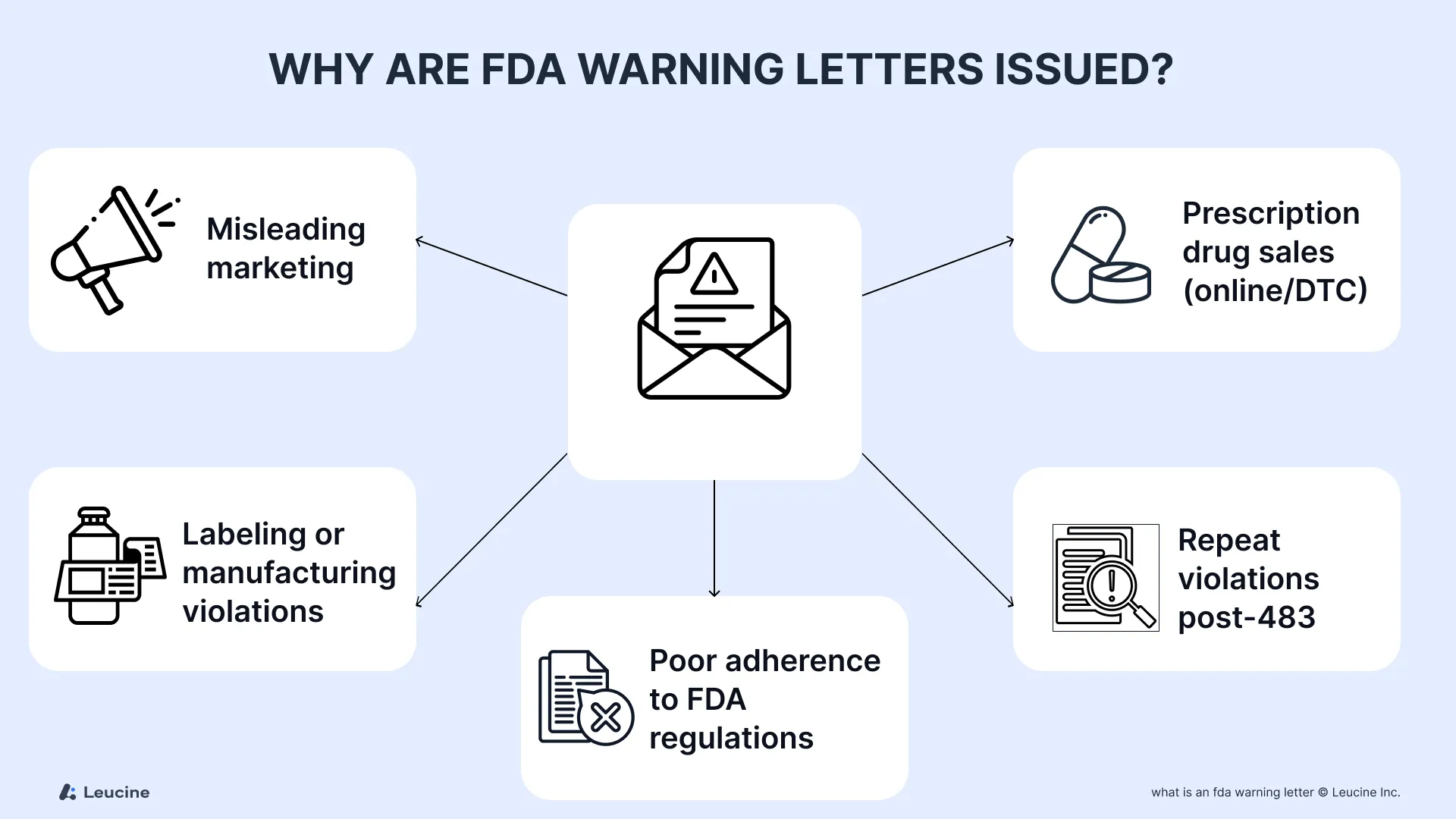
Common triggers include:
- Poor adherence to FDA regulations or implementing regulations
- Misleading statements on web sites, marketing content, or social media (covered under advertising warning letters)
- Attempts to sell online prescription drugs or conduct illegal prescription drug sales
- Manufacturing and labeling violations in drug marketing
- Repeat violations found in follow-up inspection or subsequent inspection
Such violations often stem from not following written procedures, failing to correct previous issues, or engaging in illegal activities that put public health at risk.
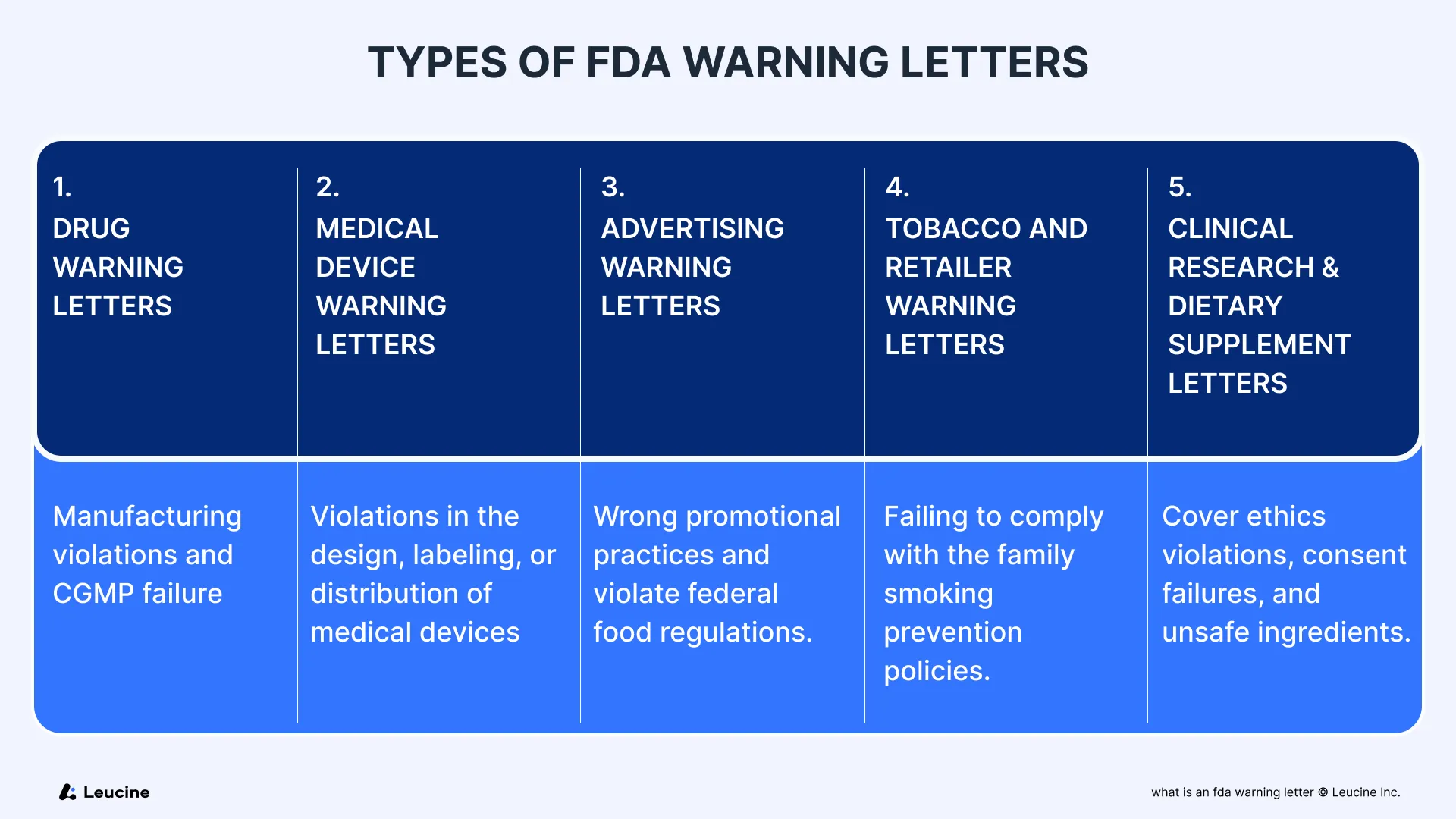
Types of FDA Warning Letters
Although the tone and structure of most general FDA warning letters are consistent, there are several specialized types, each addressing specific risks and requests from the agency:
1. Drug Warning Letters
Issued for violations related to manufacturing, process validation, and failure to meet current Good Manufacturing Practices. These are common in prescription drug sales or cases involving active pharmaceutical ingredients.
2. Medical Device Warning Letters
Target violations in the design, labeling, or distribution of medical devices, especially if patient safety is at risk.
3. Advertising Warning Letters
Focus on confidential information, unsubstantiated health claims, or promotional practices that govern prescription drug sales and violate federal food regulations.
4. Tobacco and Retailer Warning Letters
Directed at tobacco retailers for selling smokeless tobacco or tobacco products to minors or failing to comply with the family smoking prevention policies.
5. Clinical Research & Dietary Supplement Letters
Cover ethics violations, consent failures, and unsafe ingredients. Also includes untitled letters for less severe issues that don’t meet the threshold of a warning letter but still require prompt action.
Consequences of Ignoring an FDA Warning Letter
Once warning letters are issued, the clock starts ticking. If your response is delayed, vague, or lacks supporting corrective action, you may face:
- Further notice of regulatory action or legal proceedings
- Application holds and delays for product approvals
- Subsequent interaction or follow-up inspections
- Permanent record of non-compliance that affects your market access
- Public posting of the letter and outcomes on FDA’s database of other warning letters
The agency considers these letters serious. A poor or late written response may trigger a final action, like an import ban or criminal prosecution.
How to Respond to an FDA Warning Letter
Your goal after receiving an FDA warning is prompt voluntary compliance. That means showing the agency that you are taking the corrective action seriously and are committed to addressing all concerns.
What your response should include:
- Point-by-point acknowledgment of the violations
- Corrective and preventive action plans (CAPAs)
- Revised written procedures and training evidence
- Proof of system-wide fixes to prevent recurrence
- Commitment to being ready for subsequent inspection
If the FDA is satisfied, they may issue a close out letter acknowledging that the matter is resolved and no further regulatory action is needed.

Be Proactive: Use FDA Tracker to Stay Ahead
Want to avoid becoming the next recipient of a drug warning letter or advertising warning letter? Leverage FDA Tracker to analyze trends, identify high-risk zones, and monitor competitors’ regulatory status.
With FDA Tracker, you can:
- Review recent warning letters issued and their outcomes
- Understand requests in different sectors of the regulated industry
- Track patterns across product categories and fiscal years
- Prepare for follow-up inspections with real-world benchmarking
Signup for Free on FDA Tracker
Final Takeaway
An FDA warning letter is more than a notice—it’s a turning point. It reflects your regulatory significance, your agency relationship, and your ability to maintain voluntary compliance.
✅ Don’t ignore the signal
✅ Respond fast and with clarity
✅ Use the right tools to prepare for certain situations




















