FDA Form 483 vs. Warning Letter: Know the Difference Before It Costs You
Learn the difference between FDA 483 and warning letters, what triggers escalation, and how to avoid regulatory action with smart compliance strategies.

In the regulated industry of pharmaceuticals and other FDA regulated products, the distinction between a Form 483 and FDA warning letters is more than just regulatory semantics—it defines your company’s regulatory status, risk exposure, and path toward sustained compliance.
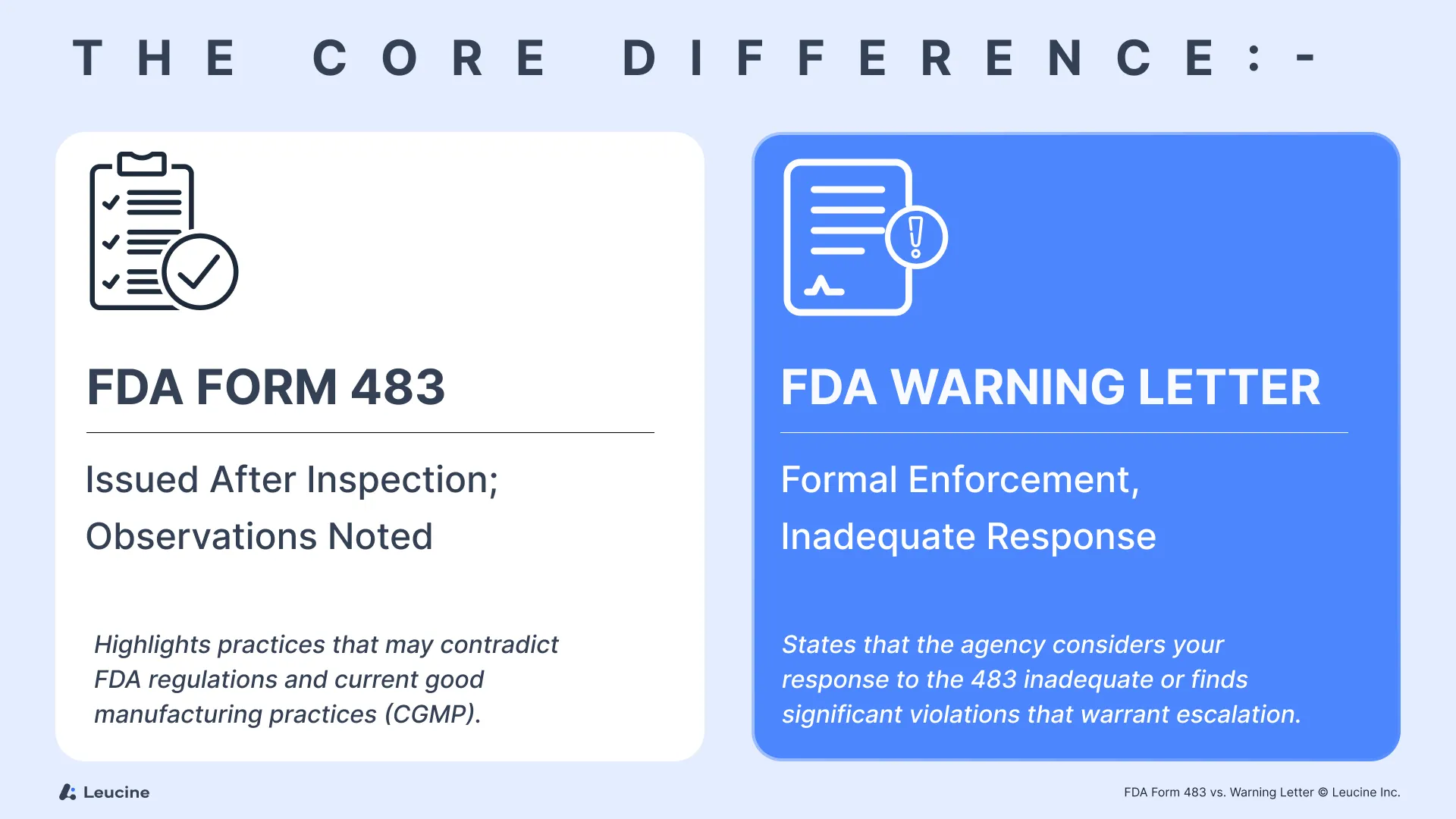
The Core Difference
- A Form 483 is a document issued by FDA investigators at the end of an inspection. It highlights practices and specific violations observed that may contradict FDA regulations and current good manufacturing practices (CGMP).
- A warning letter—or more precisely, FDA warning letters—is a formal enforcement action that states the agency considers your response to the 483 inadequate or finds significant violations that warrant escalation.
Think of a Form 483 as a serious advisory and a warning letter as an escalation with potential legal consequences, including the ability to withhold approval of drug applications or medical devices, especially Class III devices or active pharmaceutical ingredients.
How One Becomes the Other
When a facility fails to take prompt corrective action following a form 483, the Food and Drug Administration may issue a warning letter. These letters warn the inspected facility that failure to correct the violations identified could lead to more severe outcomes such as injunctions or import alerts.

Triggers include:
- Lack of supporting information in the written response
- Delayed or vague corrective action plan
- Repeated violations related to written procedures, process validation, or quality control
- Issues tied to clinical trials, drug product handling, or medical devices
These letters can even cite online web sites or prescription drug sales as potential violations, especially in e-commerce or DTC models.
Explore real FDA 483 warning letters and their escalation paths for free
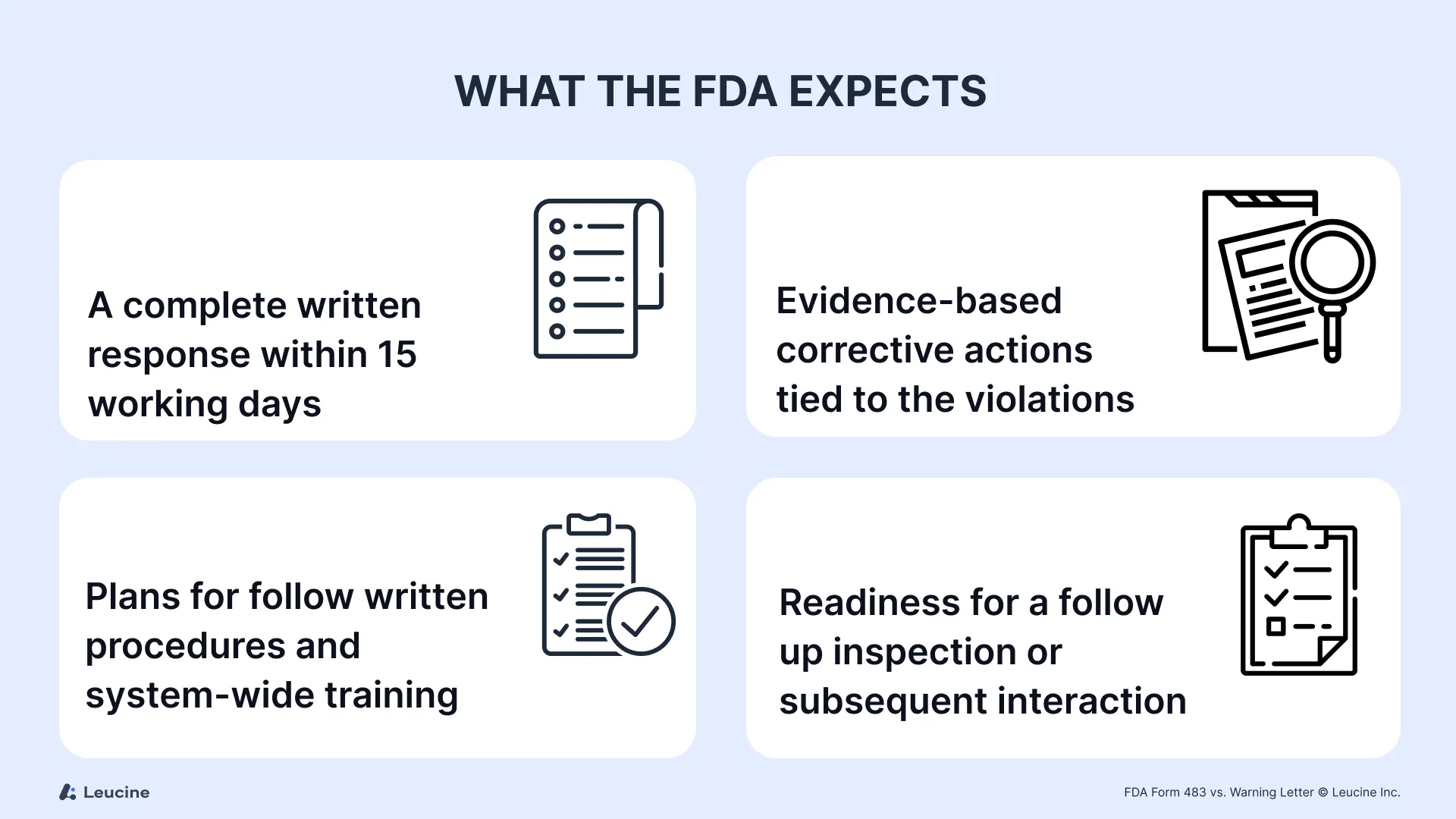
What the FDA Expects
Whether it’s a FDA form 483 or a drug warning letter, the agency expects:
- A complete written response within 15 working days
- Evidence-based corrective actions tied to the violations
- Plans for follow written procedures and system-wide training
- Readiness for a follow up inspection or subsequent interaction
A failure to demonstrate commitment can result in regulatory action, restricted product approvals, and major delays—especially for FDA regulated products.
In some cases, the agency may issue a close out letter following a successful subsequent inspection if all violations are corrected and compliance is achieved.
Stay Ahead with FDA Tracker
Smart companies don’t wait for the FDA’s concerns to escalate—they monitor industry trends to prevent them.
FDA Tracker offers:
- Access to thousands of FDA 483 warning letters
- Filters for fiscal year, product type, and inspection region
- Insight into how warning letter identifies serious violations
- Benchmarking tools to assess your regulatory status
- Alerts on recent CGMP violations or potential regulatory violations
Signup for Free on FDA Tracker
Final Thoughts
In the eyes of the agency, a Form 483 is a red flag. A warning letter is a regulatory crossroads. Both carry potential consequences, but the outcome is determined by how your company responds.
✅ Own the issues discussed
✅ Address the violations early
✅ Use tools like FDA Tracker to stay ahead of potential regulatory action




















