How Electronic Batch Record systems are transforming Pharma Manufacturing

What is an Electronic Batch Record (eBMR)?
An Electronic Batch Record (eBMR) is a digital version of the traditional Batch Manufacturing Record (BMR). It captures every detail of the manufacturing process—from raw material dispensing to final packaging—ensuring real-time data integrity, traceability and regulatory compliance through robust compliance procedures. Digital solutions play a key role in data collection and operational efficiency, giving manufacturers a competitive edge.
Unlike paper based batch records which are manual, error prone and difficult to scale, eBMRs are dynamic electronic record systems. These digital records not only improve quality but also ensure audit readiness by complying with regulations such as FDA and 21 CFR Part 11. They provide complete visibility into every step of the batch production record, aligned to standardized process controls and global cGMP requirements. Integrated document management systems streamline the creation, storage and retrieval of documents, securing critical batch records.
Explore how BMR guidelines apply to your digital strategy
Why the Shift from Paper to eBMR?
Batch records have been a part of pharmaceutical manufacturing for long. However the shift to digital is driven by need for operational efficiency, audit readiness and better production quality. Also moving to cloud raises important questions about data security, cloud providers like Microsoft Azure and AWS are more secure and compliant than traditional data centers. To achieve this many companies are opting for electronic batch records which streamlines documentation, reduces errors from manual processes and compliance to regulatory standards.
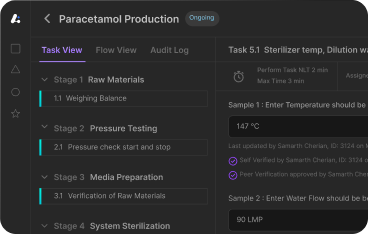
%20_%201.webp)
Using digital in manufacturing and pharma industry makes efficiency and compliance by replacing traditional processes with digital solutions like electronic batch record (EBR) systems.
Limitations of paper records are:
- Manual entries prone to human error
- Batch record reviews are cumbersome
- Delays in data collection and approvals
- Risk of non-compliance due to missing or incorrect documentation
Electronic batch records addresses these pain points by giving:
- Real time data collection
- Integrated quality control and validation checkpoints
- Instant access for cross functional review
- Strengthened process control systems and compliance
Learn how digitized batch release aligned with FDA expectations helps reduce cycle times.
See how eBMR accelerates batch release
Core Features of eBMR Systems
An advanced Electronic Batch Record (eBMR) system is more than a paperless replica—it’s an integrated digital command center for pharmaceutical manufacturing. The right electronic batch records solution addresses challenges associated with manual management of batch records, such as data integrity, human error, and compliance issues. The right eBMR platform, often referred to as an ebr software system, supports intelligent batch execution by automating documentation, standardizing workflows, and embedding compliance into every step of the process. This ebr software system also plays a crucial role in eliminating bottlenecks in traditional batch manufacturing, automating compliance, and detecting risks, which enhances efficiency and ensures timely batch release. A powerful electronic batch record system is more than a digital checklist—it’s a foundation for smart, scalable batch manufacturing processes. Here are the key features:
Enterprise resource planning systems are essential tools that facilitate the efficient management of batch records, ensuring adherence to regulatory standards and providing comprehensive oversight of production processes.
Automated Data Capture
Connects with equipment and systems to eliminate transcription errors and ensure accurate, time-stamped entries for every batch by capturing data from various sources including sensor data. The system reviews user input in real time to ensure consistency and accuracy of data collection. Operators in the lab interact with various devices and instruments that produce critical process data including sensor data and quality control readouts.
Workflow Integration
Integrates with manufacturing execution systems (MES), QMS, LIMS and other enterprise systems to centralize information, capture process deviations and link directly with quality control protocols.
Version Control & Traceability
Maintains full audit trail and change history, simplifies batch record review and audit readiness.
Electronic Signatures
Compliant with 21 CFR Part 11, allows secure, authenticated sign-offs by authorized personnel.
Real-Time Visibility
Provides QA and production teams with access to live execution and production data, improves response times and supports agile production processes.
%20_%202.webp)
Leucine’s eBMR-enabled MES platform is built for life science companies. It supports rapid deployment, configurable workflows and built-in compliance for complex batch records digitization.
Real-World Impact of eBMR Adoption
Example: Pharmaceutical companies, such as a sterile injectable plant, improved operational efficiency in their batch manufacturing process, reducing batch release time from 5 days to under 24 hours by adopting eBMR with real-time batch manufacturing record validation and exception-based reviews. Maintaining accurate batch numbers is also crucial for compliance with FDA and EMA guidelines.
Use cases where eBMR systems drive value include:
- Sterile and high-volume production environments
- Regulatory inspections and audits
- Enabling remote batch record review during global scale-up, with release tests assuring stakeholders and regulatory agencies of product quality and safety
Overcoming Implementation Challenges
Moving from traditional batch documentation to a fully digital electronic batch record system offers long-term benefits—but getting there requires planning and cross-functional alignment. For many life sciences companies, especially those in life sciences manufacturing, the challenge isn’t the technology—it’s the change. Adhering to good manufacturing practices is key to compliance, product safety and quality standards.
1. Cultural and Training Barriers
One of the biggest hurdles in implementing electronic batch records is user adoption. Teams used to paper based batch records may resist change. That’s why successful eBMR implementation starts with comprehensive, role-specific training designed to show not just how to use the system—but why it matters. Bridging this cultural gap is critical to building digital maturity across manufacturing, quality and compliance teams.
2. Validation and Regulatory Compliance
Every eBMR solution must be validated according to GxP guidelines and 21 CFR Part 11 requirements to ensure compliance with regulatory standards. The validation process can seem daunting but modern systems simplify it through automated test protocols, version control and pre-configured templates aligned to FDA and EMA expectations. These systems not only provide data accessibility but also data integrity which is critical for production quality and adhering to strict regulations in manufacturing and pharma. A trusted provider will guide you through this phase to be audit ready from day one.
3. Migration and Integration
Digitizing batch records isn’t just about going paperless—it’s about creating an integrated ecosystem. That includes migrating master batch records, linking to QMS and MES platforms and ensuring batch manufacturing processes are mirrored correctly in the new system. Ensuring SOP alignment and production continuity during rollout is key to success.
4. Scaling Across Sites and Teams
Scalability is key in the life sciences industry. Many companies start digitization at one site and then face challenges when rolling out to multiple sites or contract manufacturers. Look for eBMR platforms that offer cloud-based deployment, modular configuration and multilingual support to make global scale-ups more efficient.
%20_%203.webp)
Tip for Success
Choose an eBMR provider that is a strategic partner—not just a software vendor. The right partner will bring more than tools: they’ll bring frameworks for digital transformation, experience with life science companies and a roadmap for continuous improvement.
How to Choose the Right eBMR Software
When evaluating an eBMR platform consider the following:
- Does it use electronic records to streamline manufacturing processes and ensure compliance:
- Scales with multi-site batch manufacturing operations
- Adapts to your existing production processes and SOPs
- Integrates with other digital systems (MES, QMS, ERP)
- Compliant with data integrity and electronic signatures
Digital solutions can improve efficiency by increasing connectivity and automating data collection which improves production processes and compliance.
Understanding these factors is key to seamless integration and optimal operation of eBMR systems in manufacturing and pharma processes including medical devices. The right platform should simplify compliance and unlock smarter manufacturing.
Conclusion: The Future is Digital
As the pharmaceutical industry accelerates into Pharma 4.0, electronic batch records are at the center of this transformation. By reducing human error, enabling real-time data collection, and supporting continuous improvement through analytics, eBMRs redefine what's possible in batch production.
Leucine empowers life science companies to implement and scale eBMR systems confidently—transforming compliance into a strategic advantage.
Start your digital batch transformation with Leucine