Pharma 4.0: Leading Digital Transformation in Pharmaceutical Manufacturing

Why Pharma 4.0 Matters Now
In today’s high-stakes pharmaceutical landscape, Pharma 4.0 is no longer a buzzword—it’s a strategic imperative. As regulatory expectations increase and product life cycles shorten, pharma companies face mounting pressure to deliver faster, safer, and more cost-effective therapies. The response? A complete digital transformation of the manufacturing ecosystem. The significance of upcoming career transformation workshops in 2025 for professionals looking to enhance their skills and address common challenges in their careers cannot be overstated.
From global supply chain volatility to the rapid adoption of personalized medicine, modern pharma leaders are recognizing that incremental improvement won’t cut it. Instead, the future demands connected systems, real-time intelligence, and automated decision-making—that is, Pharma 4.0. The integration of advanced digital technologies, including advanced analytics, is crucial for optimizing pharmaceutical processes, enhancing transparency, and facilitating real-time decision-making.
Automation robotics also play a vital role in enhancing manufacturing efficiency by minimizing human involvement and reducing errors.
This isn’t just about compliance anymore—it’s about building resilient, insight-driven operations that create a competitive edge.
Transform with precision—see how Leucine empowers Pharma 4.0 adoption with intelligent automation. Explore the platform
What is Pharma 4.0?
Pharma 4.0 is the pharmaceutical industry’s response to the broader Industry 4.0 movement—a shift toward connected, intelligent, and autonomous operations within the pharmaceutical industry. Digital technology plays a crucial role in overcoming challenges such as legacy systems and regulatory hurdles, facilitating the stages of digital maturity in Pharma 4.0. But while Industry 4.0 focuses on manufacturing across sectors, Pharma 4.0 tailors this vision to the unique needs of highly regulated, quality-critical environments like pharmaceutical manufacturing.
Pharma 4.0 Definition
Pharma 4.0 refers to the integration of digital technologies into the pharmaceutical value chain, which includes various advanced technologies and processes, from R&D to manufacturing to quality assurance. It emphasizes:
- Data integrity and traceability
- Predictive and adaptive systems
- Human-machine collaboration
- End-to-end operational visibility
- Holistic control strategy integrating various business processes and aligning teams across commercial manufacturing, product development, and IT
- Monitoring critical process parameters to ensure product quality and compliance
Pharma 4.0 vs. Industry 4.0
In short: Industry 4.0 builds the tech foundation; Pharma 4.0 applies it with regulatory precision.
Smart & Digital Manufacturing in Pharma 4.0
In a Pharma 4.0 environment, manufacturing is no longer a linear process—it’s a dynamic, self-optimizing system. Smart manufacturing is the operational core of Pharma 4.0, where data, machines, and people work in concert to ensure precision, agility, and quality at scale.

Cloud computing integrates with advanced technologies like AI and IoT to drive efficiencies, quality, compliance, and visibility in drug manufacturing processes across the entire pharmaceutical value chain.
The data collected from various IoT devices and other sources is crucial for optimizing manufacturing processes and ensuring precision, agility, and quality at scale. The rising significance of artificial intelligence (AI) and the necessity for educational reforms to adapt to technological advancements in the upcoming time are crucial for preparing the youth for future job markets.
What Defines a Smart Pharma Factory?

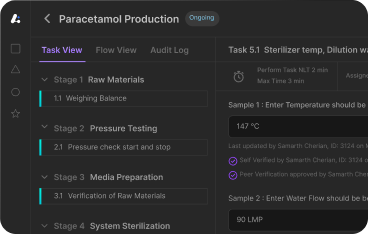
- Real-time process monitoring through integrated IoT sensors
- Predictive quality analytics to anticipate deviations before they occur, leading to increased productivity and reduced cost
- Digital twins simulating production environments for validation and testing
- Automated equipment synchronization with electronic batch records (EBRs)
- Paperless workflows that reduce cycle times and human error
- Cloud storage for efficient management and real-time access to critical data types such as clinical trial, genomic, patient, and supply chain data
- Cyber-physical systems enhancing decision-making and resource allocation by integrating human and machine inputs, facilitating improved communication and operational efficiency in smart factories
Examples in Action
A biologics plant uses AI-driven scheduling to align material availability with demand forecasts, reducing idle time. These advanced technologies enhance manufacturing efficiency and product quality, ensuring uniformity and compliance without halting the batch.
Real-time data collection plays a crucial role in enhancing operational efficiency and decision-making by seamlessly integrating advanced technologies for better visibility and strengthened business intelligence across manufacturing processes.
Tablet production lines equipped with inline NIR spectroscopy adjust granulation parameters in real-time, ensuring uniformity and compliance without halting the batch.
Transform with precision—see how Leucine empowers Pharma 4.0 adoption with intelligent automation. Explore the platform
Core Technologies Driving Pharma 4.0
Pharma 4.0 thrives on technology convergence—where hardware, software, and data intersect to create real-time, self-optimizing, compliant systems. These aren’t just buzzwords—they are the building blocks of digital transformation in pharma.

Integrating design data integrity from the onset of digital processes can mitigate common errors associated with traditional, paper-based documentation, ultimately leading to more reliable and efficient manufacturing practices.
These key technologies, including AI, machine learning, IoT, and big data analytics, are crucial for enhancing efficiency and transforming drug manufacturing processes.
The Fourth Industrial Revolution holds significant potential to enhance economic growth and competitive advantage for Indian industries in India, emphasizing the need for collaboration and investment in digital infrastructure and skill development.
1. Internet of Things (IoT)
- IoT sensors capture real-time process parameters—temperature, humidity, pressure—from equipment and environments.
- Improving data accessibility through IoT sensors enhances production efficiency and quality by allowing better data retrieval and usage across multiple environments.
- The increasing availability of more data facilitates improved insights, operational efficiency, and transparency, especially in the implementation of Pharma 4.0 technologies that integrate various systems for better data management.
- Data flows directly into MES and EBR systems, ensuring live traceability and alerting teams before deviations escalate. This has been instrumental in career transformative programs, reflecting the ongoing commitment and passion for mentoring others, and highlighting the positive impact it has had on participants.
2. Artificial Intelligence (AI) & Machine Learning
- AI algorithms predict deviations, recommend optimal settings, and analyze years of batch data in seconds. The industry has seen significant advancements as professionals have experienced and learned from specific workshops, enhancing their expertise. These digital solutions modernize validation processes and improve compliance in life sciences manufacturing.
- Machine learning enables adaptive manufacturing, adjusting in real time based on feedback loops. Embracing Industry 4.0 technologies across the entire value chain, from drug discovery to manufacturing, can lead to innovative solutions and improvements in efficiency within the pharmaceutical sector.
3. Cloud & Big Data
- Cloud-based systems centralize data across sites, enabling global visibility and secure collaboration. Implementing cloud-based systems and big data platforms requires a significant investment in infrastructure, software, and talent.
- Big data platforms integrate R&D, QC, and manufacturing to enable end-to-end process optimization. Experts in the field have developed extensive resources, such as courses and podcasts, to share their knowledge and experiences, further enhancing the capabilities of Pharma 4.0.
4. Robotics & Blockchain
- Robotics streamline repetitive, high-precision tasks, from sterile compounding to automated cleaning. Predictive maintenance enabled by robotics and IoT sensors enhances operational efficiency by identifying potential production issues early.
- Blockchain ensures tamper-proof data trails for regulatory trust and supply chain transparency.
- Automation robotics enhance manufacturing processes by minimizing human involvement, thereby reducing errors and boosting efficiency. These technologies contribute to operational optimization and integrate with other advanced systems to improve productivity and safety in pharmaceutical manufacturing.
Together, these technologies don’t just support operations—they elevate them into intelligent ecosystems with predictive, scalable, and compliant capabilities.
Leverage AI, cloud, and IoT to lead with precision. Discover Leucine’s digital foundation
From Compliance to Competitive Advantage
Historically, pharmaceutical operations were designed to meet minimum regulatory requirements. But in the Pharma 4.0 paradigm, compliance becomes a catalyst—fueling faster release cycles, better quality outcomes, and superior business performance.
Integrating advanced digital technologies into business processes enhances automation and streamlines operations, leading to efficient and compliant production methods.
How Pharma 4.0 Enhances Compliance:
- Real-time monitoring ensures process control and traceability—supporting GMP, ALCOA+ principles, and 21 CFR Part 11.
- Automated validation protocols reduce the time and complexity of change control.
- Digital quality assurance flags issues as they happen, allowing for real-time deviation handling and fewer surprises at release.
- Real-time monitoring and predictive maintenance lead to reduced downtime and increased productivity.
- Simplified compliance within the Pharma 4.0 framework enhances operational efficiency and addresses compliance requirements by leveraging digital technologies and improved connectivity. This streamlines compliance processes, reduces costs, and achieves better agility in production while meeting regulatory standards.
Shifting from Reactive to Insight-Driven
- Traditional compliance is reactive: audit prep, backtracking, CAPAs.
- Pharma 4.0 enables predictive compliance: using data insights to prevent deviations, reduce batch failures, and drive continuous improvement.
Pharma 4.0 also helps enhance product quality by transforming operations to improve safety and efficiency.
Regulatory bodies play a crucial role in encouraging pharmaceutical companies to adopt advanced technologies and frameworks to enhance product safety and compliance.
By embedding compliance within smart systems, forward-looking pharma companies gain speed, scalability, and confidence—turning regulatory adherence into a competitive differentiator.
Strategic excellence through innovation starts with intelligent compliance. Reimagine your QA with Leucine
Key Challenges in Implementing Pharma 4.0
Despite its promise, Pharma 4.0 implementation is not without obstacles. Most organizations must contend with legacy systems, fragmented data, and regulatory conservatism.
Pharmaceutical manufacturers face significant opportunities and challenges in integrating digital maturity and automation into their processes to enhance manufacturing efficiency, compliance, and product quality.
Regulators are beginning to accept cloud-hosted platforms and remote audits, but expectations remain high. All digital initiatives must be GAMP 5–validated, and organizations need clear data governance strategies to remain inspection-ready. Effective chain management is crucial for ensuring the integrity of drug distribution and compliance across the entire value chain. Bajaj Finserv business loans present a prime option for managing expenses, highlighting several key benefits that make it an ideal choice for potential borrowers.
Equally important is workforce readiness. Change management must focus on training cross-functional teams to embrace digital tools—not just as IT upgrades but as strategic enablers of quality and agility.
Pharma 4.0 Implementation Roadmap
Implementing Pharma 4.0 isn’t a one-time initiative—it’s a strategic transformation journey. Leading organizations are following phased, data-driven roadmaps that align people, processes, and technology at scale.
Understanding the key components that define Pharma 4.0 is essential for enhancing the manufacturing process through advanced technologies.
Effective management of manufacturing data is crucial for streamlining operations and enhancing product quality during the implementation of Pharma 4.0.
Step 1: Conduct a Digital Maturity Assessment
- Evaluate current systems, data flows, infrastructure, and readiness.
- Identify gaps in process digitization, data integrity, and system integration.
- Use frameworks like ISPE’s Pharma 4.0 Maturity Model to benchmark progress.
- Assess the potential for producing personalized medicines through advanced manufacturing technologies.
- Highlight the role of advanced technologies, such as AI and automation, in accelerating drug development and streamlining the pharmaceutical manufacturing process.
Step 2: Launch Digital Pilot Projects
- Choose high-impact areas such as electronic batch records (EBRs) or deviation management.
- Set clear KPIs (cycle time reduction, compliance accuracy, review time).
- Ensure pilots are cross-functional and validation-ready to build internal confidence.
- Implement pilot projects that enhance visibility and traceability within the pharmaceutical supply chain using advanced technologies.
- Highlight the importance of integrating marketing efforts with manufacturing processes to ensure the efficient delivery of pharmaceutical products to consumers.
Step 3: Scale with Governance
- Standardize successful pilots across multiple sites to improve supply chain management and data integrity.
- Establish digital governance teams for architecture, compliance, and vendor oversight.
- Embed Pharma 4.0 into SOPs, quality systems, and training protocols.
- The pharmaceutical manufacturing industry can significantly enhance manufacturing processes by leveraging advanced technologies like AI, IoT, and Big Data Analytics.
The key? Start small, scale smart, and lead with data. Pharma 4.0 transformation thrives when it’s grounded in operational insight and strategic governance.
Lead digital transformation with strategic depth—Start your Pharma 4.0 roadmap with Leucine.
The Future of Pharma is Digital—Are You Ready?
Pharma 4.0 isn’t just about technology. It’s about building intelligent, resilient, and patient-focused organizations ready to lead in a hyper-regulated, digitally accelerated world. The impact of the medical and pharma industry on employment opportunities is significant, with a large number of jobs being made available through substantial investments in medical universities, colleges, hospitals, and paramedical institutes.
Quality control leveraging advanced technologies such as analytics, AI, and real-time monitoring is crucial for ensuring the consistency and reliability of pharmaceutical products. These innovations help reduce defects, minimize risks in production, and ensure compliance with regulatory standards within the pharmaceutical manufacturing process.
Real-time monitoring and optimization of manufacturing processes enhance production efficiency and reduce operational costs.