Unlocking Pharma MES for Modern Manufacturing Excellence

What is Manufacturing Execution Systems in Pharma?
Manufacturing Execution Systems (MES) are crucial in the pharma industry, enabling real-time control of production processes. As the digital layer between ERP systems and shop floor operations, MES ensures every step of manufacturing within a company is executed correctly, logged correctly and compliant to regulations, hence more efficient. This is especially important for biotech companies where MES boosts operational efficiency and regulatory compliance, that’s why you see MES everywhere in these companies.
Unlike ERP or LIMS which focus on planning or lab workflows respectively, MES is responsible for executing the process itself. It manages tasks, materials, equipment and personnel—providing end to end traceability from raw material to finished product. For those asking what is MES in pharma, the answer is simple: it’s the engine that ensures every product is made right the first time.
To strengthen your GMP compliance, start with our comprehensive resource on FDA-aligned batch records and how to structure them for audit readiness.
Evolution of Pharma MES
The development of manufacturing execution systems MES started in the 80’s when the pharma industry needed more robust documentation and regulatory compliance. Industry events like the benchmark calendar event in Europe have played a big role in discussing the advancements and challenges in MES technology. The evolution of MES has been driven by the integration of advanced technologies like generative AI and complex LLM models that enhance operational performance and support agile manufacturing. The introduction of 21 CFR Part 11 formalized electronic records and audit trails and MES evolved from SCADA/HMI tools into full scale platforms supporting GMP compliant digital transformation.
Modern MES platforms:
- Capture and validate real-time data
- Replace paper-based processes and logs with electronic batch records (EBRs)
- Integrate with ERP, QMS and LIMS systems
- Offer mobile-first, cloud-native deployments
Spot FDA risks in Batch Records before they escalate — act now.
Core Functions and Capabilities of Pharma MES
At its core a Manufacturing Execution System (MES) is designed to manage every aspect of pharmaceutical manufacturing processes—from recipe enforcement to real-time decision making. MES enables direct execution of production orders, real-time automated handling and quick adjustments to changes in orders, machine conditions and quality checks. Its value is not just in replacing paper but in creating a digital foundation where compliance, traceability and productivity work together. It’s a digital operations engine for the pharma industry. From enforcing recipes to regulatory compliance, MES brings all aspects of production into one ecosystem.
At its foundation MES governs every batch from start to finish. It allows manufacturers to enforce standardised processes, digitise documentation and get real-time visibility into the manufacturing process. These manufacturing systems help pharma companies not only improve productivity but also operational excellence in highly regulated environments. This level of control is not just efficient, it’s necessary for 21 CFR Part 11, EU Annex 11 and global GMP guidelines. Here’s what a pharma grade MES enables:
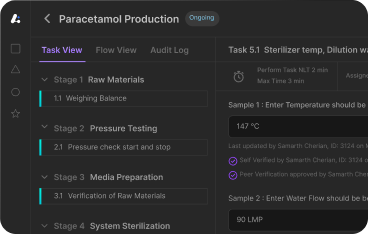
- Electronic Batch Records (EBRs) - At the heart of every MES is the ability to generate and execute electronic batch records. These records capture every action, material and operator input in real-time—providing traceability, compliance to GMP and replacing time consuming paper based batch documentation.
- Master Batch Record (MBR) Management - Master Batch Records (MBR) are digital blueprints that define how each product is to be manufactured. MES platforms create, manage and validate master batch records MBR across different SKUs and facilities—ensuring standardised, compliant batch execution regardless of where production is done. These templates also drive consistency and enable version controlled updates as products evolve.
- Real-Time Data Collection and Monitoring- MES systems integrate with shop floor equipment, sensors and PLCs to automatically collect data—temperature, pressure, weight, quality checks—during production. This digital manufacturing capability enables proactive deviation management, continuous process verification and real-time visibility for supervisors and QA teams.
- Resource and Inventory Management- MES tracks material consumption, equipment usage and even personnel training records. This ensures only qualified users perform designated tasks and materials meet quality control requirements before use. It also minimizes inventory wastage and supports planning.
- Deviation Tracking and Audit Trails- Deviations, exceptions and critical process alarms are logged and routed for review within the MES. Each activity is time-stamped and linked to specific users—creating a complete audit trail for internal investigations and external audits.
- Workflow Automation and Digital Signatures- Manual processes are replaced with guided digital workflows. At key checkpoints users apply secure electronic signatures compliant with 21 CFR Part 11, ensuring accountability and traceability.
- Integration with QMS, LIMS, and ERP Systems - For a true connected manufacturing environment MES must integrate with existing business systems. This includes two-way communication with ERP for production orders, LIMS for test results and QMS for CAPAs or audit documentation. These integrations eliminate silos and reduce batch disposition and release delays.

Together these capabilities make MES not just a compliance tool—but a strategic enabler of leaner, smarter and more agile pharmaceutical operations.
Explore how Leucine’s MES brings these capabilities together
Ensuring Regulatory Compliance in the Life Science Industry
In the highly regulated life science industry, ensuring regulatory compliance isn’t optional—it’s mission-critical. From global GMP standards to region-specific mandates like 21 CFR Part 11 in the U.S. and EU Annex 11 in Europe, life sciences companies are required to maintain rigorous documentation, traceability, and data integrity across their manufacturing operations. This is where a robust Manufacturing Execution System (MES) proves indispensable.
Pharma-grade MES platforms help companies enforce compliance at every stage of production. They automate documentation, enforce standard operating procedures (SOPs), and generate comprehensive audit trails that align with regulatory guidelines. This eliminates reliance on manual data entry and paper-based records, significantly reducing the risk of human error and non-compliance.
Moreover, with built-in capabilities for electronic signatures, deviation tracking, and real-time monitoring, MES enables QA teams to proactively identify and resolve issues before they escalate. By digitizing these critical compliance functions, MES empowers pharmaceutical manufacturers to stay ahead of audits, accelerate batch reviews, and maintain consistent product quality—key imperatives in the life science industry.
Whether you're manufacturing biologics, generics, or personalized therapies, adopting an MES ensures your operations remain inspection-ready and aligned with evolving global regulations—ultimately protecting patient safety and brand reputation.
Strategic Benefits of Pharma MES
Beyond automation MES delivers measurable business impact in the pharma industry by reducing costs and improving operational efficiencies. With increasing market pressures organisations are forced to improve product quality while reducing costs and improving operational efficiencies. Market pressures push the life science industry to adopt innovative and sustainable manufacturing solutions to navigate these challenges effectively. It enables manufacturers to build sustainable competitive advantages through:
- Faster batch reviews and release
- Significant reduction in human error
- Better visibility into product quality, yield and production schedules to improve overall efficiencies
- Enhanced collaboration across production, QA and supply chain
- Built-in regulatory documentation to pass audits with confidence
Want to ensure your digital batch records are inspection-ready?
This is especially valuable when aligning batch release procedures with FDA expectations, where delays or manual reviews can be costly.
Deployment Models: One Size Doesn’t Fit All

Every pharmaceutical company has different infrastructure and IT requirements, including the need to manage both shop floor operations and office based functions. MES platforms come in three main deployment options:
- On-Premise MES: High control, suitable for organisations with strict data residency needs, full control over data management and security.
- Cloud-Based MES: Agile, cost effective and perfect for scaling across sites and regions.
- Hybrid MES: Combines local execution with cloud synchronisation—ideal for global life science industry rollouts.Leucine’s cloud MES is designed for companies looking to reduce manufacturing costs and improve scalability and compliance.
Leucine’s cloud-native MES is built to serve companies looking to lower manufacturing costs while improving scalability and compliance.
Understanding Terminology in Context
MES isn’t always called “MES”. Depending on the vendor or business unit it may be called an MES system, EBR platform, MOMS (Manufacturing Operations Management System) or simply a Digital Manufacturing System. MES often integrates with enterprise resource planning (ERP) systems to improve operational efficiency and streamline production processes. The functionality overlaps but not all platforms are purpose-built for pharmaceutical operations.
That’s why it’s essential to choose a solution that aligns with your team’s workflows, regulatory requirements and digital maturity.
Choosing the Right Pharma MES
Selecting an MES isn’t just about ticking off a features checklist. It’s about aligning software with production processes, regulatory expectations and business goals to streamline operations.
Before committing pharma leaders should ask:
- Does the MES comply with GAMP5, 21 CFR Part 11 and EU GMP?
- Can it integrate with my existing ERP and quality systems?
- Will it scale from pilot to multi-site deployment?
- Can it replace my legacy systems and reduce compliance risks?
- Does the MES support robust data management architectures to ensure data integrity and seamless integration with other systems?
Mes and Pharma 4.0

It’s not just about execution—it’s about smart, connected manufacturing. Engaging with industry experts can provide valuable insights and best practices for leveraging MES to its full potential. The benefits of MES are not just operational—they’re strategic. By embedding intelligence into every batch, MES transforms how pharmaceutical teams manufacture, review, and release products. MES systems can react dynamically to changing orders, machine statuses, and quality checks, enhancing overall productivity and responsiveness. This focus on manufacturing excellence ensures that advanced technologies and intelligent manufacturing solutions are adopted, leading to rapid improvements in product delivery and operational performance.
MES helps reduce batch review cycles from days to hours, minimizes manual errors, and strengthens right-first-time performance. QA teams gain full visibility into live execution, and deviations are flagged and resolved before they become compliance risks. With MES at the core, manufacturers can:
- Implement predictive maintenance through IIoT
- Analyze process performance in real time
- Enable continuous improvement through closed-loop feedback
- Connect to data lakes for AI-driven insight generation
It also elevates audit readiness. Every signature, exception, and correction is tracked with time-stamped accuracy, providing a digital trail that’s easy to retrieve during inspections.
Future Trends in MES for Pharma
As the pharmaceutical industry and the broader life sciences industry evolve, so will MES. Events in Europe dedicated to Pharma and Bio-tech manufacturing will continue to play a crucial role in shaping these trends. Expect to see:
- More mobile-friendly interfaces for operators and supervisors
- Greater modularity to accommodate complex production processes
- Expanded support for personalized medicine and small-batch biologics
- Expanded support for biotech companies and small-batch biologics
- Cloud-based, AI-augmented MES systems built for continuous innovation
Future-ready MES platforms will help pharma teams drive quality, compliance, and innovation at scale.
Conclusion: MES is the Foundation of Digital Pharma
Whether you’re modernizing from spreadsheets or scaling globally, implementing an MES designed for the pharma and biotech manufacturing process is essential. It unifies batch records, production execution, and compliance monitoring into a single, intelligent system.
At Leucine, we help life sciences companies transition from fragmented processes to connected, data-driven manufacturing.
Start your MES transformation with Leucine