Batch Production in Pharmaceuticals - Strategic Excellence Through Innovation

Introduction to Batch Production
In pharmaceutical manufacturing, batch production is more than a legacy model—it’s a strategic manufacturing process that enables precision, compliance, and rigorous quality control. Unlike batch production, continuous and flow production operate without interruptions, allowing for a consistent and efficient output. The batch production process segments operations into discrete, traceable units—ideal for high-value, low-volume drugs.
This flexible approach supports various production methods, allowing companies to balance agility with control. Manufacturing batch production continues to play a pivotal role in driving compliance and scalability in the pharma landscape.
Explore how batch release frameworks ensure compliance and agility
Key Characteristics of Batch Production Process
What distinguishes batch production from other production methods is its structured, controlled, and compliance-centric approach. Each batch represents a self-contained manufacturing cycle—planned, executed, documented, and reviewed as a discrete unit. This design not only supports stringent quality control measures but also simplifies regulatory validation. When comparing batch production vs other methods like mass production and continuous production, the key differences lie in production scale, setup times, and customization potential.
One defining feature is the defined quantity of output. A batch is produced using fixed formulations and parameters, ensuring every unit within that batch maintains consistent identity, strength, and purity. This homogeneity is essential in industries like pharma, where even slight variations can affect therapeutic outcomes.
Batch production also operates in fixed cycles—allowing for pause points between batches, and often utilizes generalist equipment that can be easily adapted to different production needs. These moments are strategically used to perform in-process checks, calibrate instruments, review documentation, and make adjustments before continuing. Unlike mass production or continuous production, this intermittent flow reduces systemic risk and enhances process control.
Products transition through various stages, each meticulously managed to ensure quality and compliance. Furthermore, batch production process environments are tightly regulated. Cleanrooms, specialized equipment, and validated utilities ensure minimal contamination risk and repeatable output. These settings are tailored to specific drug types, making the process scalable for small production runs or highly potent compounds.
However, the initial outlays for specialized equipment and technology can be higher compared to other production methods. Today, modern batch manufacturing integrates real-time data capture and analytics. Platforms like Leucine enable teams to monitor every stage—linking operator input, equipment data, and material traceability—making batch production not just a method, but a competitive advantage.
Explore how Leucine enables traceable, controlled batch production
Batch Manufacturing Process Workflow in Pharma
A well-orchestrated batch production process typically includes:
- Pre-production planning – raw material readiness and documentation
- Weighing and dispensing – accurate input measurement
- Manufacturing – blending, granulation, compression, coating
- Packaging and labeling – compliance-ready identification
- Storage and dispatch – controlled release and warehousing
Integrating maintenance activities into the batch production schedule can minimize production delays and ensure equipment remains in optimal condition.

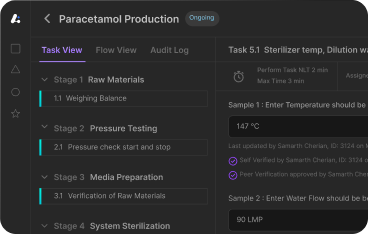
Each of these stages is logged within the Batch Record, ensuring traceability and enabling effective deviation management. This workflow enables compliance, visibility, and greater quality control, forming the backbone of batch manufacturing process documentation and review. Effective quality assurance practices are embedded at each stage, ensuring that any deviations are promptly identified and addressed.
Regulatory Guidelines and Compliance Expectations
Regulators like the FDA and EMA demand robust adherence to batch process manufacturing protocols. This includes:
- Complete documentation of the entire batch lifecycle
- Proven equipment qualification and cleaning procedures
- Strong data integrity controls within the production line
Successful audits hinge on how well a company manages these expectations—and how well their systems support them.
Check 2025 guidelines on Batch manufacturing : Batch Manufacturing Record Guidelines in the Pharmaceutical Industry
Advantages of Batch Production in Pharma
Batch production offers unmatched visibility and control, enabling pharma manufacturers to isolate issues, enforce corrective actions, and protect product integrity. The segmented nature of batch operations means that when a deviation occurs, it is contained within a specific lot, simplifying investigation and remediation. Batch production also offers significant cost savings by enabling the production of multiple items simultaneously, reducing material usage and labor requirements. Batch production remains the model of choice for companies that prioritize quality and control. Its advantages include:
- Clear traceability across production cycles
- Rapid containment of deviations within a specific batch
- Operational flexibility for diverse formulations
- Easier integration of automated quality control checkpoints
While batch production is efficient, it may not always meet specific demand for unique items tailored to individual customer requests.
Batch production is particularly advantageous for producing large quantities of items efficiently, catering to high demand while maintaining quality and flexibility. In short, it enables pharmaceutical teams to transform with precision—delivering safe, effective, and compliant therapies.
Disadvantages of Batch Production in Pharma
- Longer Production Time: Each batch requires setup, testing, and validation, which can delay overall production timelines compared to continuous manufacturing.
- Higher Inventory Costs: Managing raw materials and finished goods in batches often leads to increased storage requirements and inventory holding costs.
- Risk of Human Error: Manual transitions between batches can introduce variability and increase the risk of mistakes if not properly controlled.
- Resource-Intensive Quality Checks: Each batch demands rigorous quality checks and documentation, consuming significant time and resources.
- Downtime Between Batches: Equipment may need cleaning, calibration, or changeovers between batches, leading to production downtime.
- Limited Real-Time Data: Quality and process control are typically assessed after batch completion, which can delay problem detection and resolution.
Challenges and Limitations
While batch production offers control and traceability, it’s not without its operational challenges—many of which impact production efficiency, cost, and scalability.
First, the nature of batch processing introduces inherent downtime. Each production run requires setup, validation, execution, and cleanup. This sequential approach can lead to prolonged idle time between production runs, especially when switching between products or formulations. In contrast to continuous production, batch methods trade throughput for traceability—often increasing overall production costs.
The reliance on manual documentation in many legacy systems remains a significant pain point. Paper-based records are not only prone to human error but also create bottlenecks in batch review and release. A missed entry or misfiled log can delay release, or worse—result in regulatory observations. These risks amplify during scale-up or when managing multiple production lines simultaneously. Human error—from incorrect weighing of raw materials to improper equipment cleaning—is a leading cause of deviations. Without digital controls or automated verification, these issues are difficult to detect in real time. This makes quality control measures reactive rather than proactive.
Moreover, logistical constraints in batch production environments—such as non-drainable vessels, limited storage space between batches, and uncoordinated scheduling—can disrupt the production schedule. These inefficiencies impact labor planning, increase material wastage, and reduce overall equipment effectiveness (OEE). Finally, one of the key disadvantages of batch production is its complexity when scaling. Managing hundreds of small production processes manually becomes unsustainable without digitisation. Teams are forced to choose between flexibility and throughput—a trade-off that limits innovation.

The solution? Digitally transform your batch manufacturing process to streamline workflows, reduce human error, and unlock scalable compliance.
Slash batch cycle time and reduce error-prone handoffs: Leverage Leucine's digital batch execution tools
Digital Transformation in Batch Production
Digital-first organizations are replacing paper-based records with electronic batch records (EBRs) and connected systems. These advancements ensure:
- Real-time oversight of production runs
- Seamless MES-QMS-LIMS integration
- Embedded review, approval, and deviation tracking
- Faster throughput with fewer compliance bottlenecks
This digital foundation supports smarter, safer, and more scalable batch production.

Best Practices for Operational Excellence
Batch production is only as strong as the systems that support it. For pharmaceutical manufacturers aiming to achieve consistent, compliant, and efficient batch execution, operational excellence is not optional—it’s strategic. Below are the essential best practices that industry leaders use to maintain high performance while navigating stringent regulatory expectations. To achieve excellence in batch production method execution:
- Align SOPs with cGMP and regulatory standards
- Calibrate all batch production equipment regularly
- Use barcoding to verify steps and materials
- Train operators continuously and document competencies
- Monitor production efficiency through analytics and review
Use our Batch Records toolkit to build smarter processes
Conclusion
Batch production remains the cornerstone of pharmaceutical manufacturing—not because it’s traditional, but because it offers unmatched control, traceability, and compliance. But in today’s fast-moving, high-stakes environment, traditional alone isn’t enough. The future belongs to manufacturers who modernize their batch operations with digital systems that are intelligent, integrated, and inspection-ready by design. At Leucine, we empower pharma teams to go beyond compliance—unlocking performance, agility, and strategic advantage with every batch.
Let’s pioneer your next phase of manufacturing—digitally, intelligently, and with purpose: Start your journey with Leucine