Equipment Qualification Audit Readiness Checklist
Ensure compliance with our audit readiness checklist for Equipment Qualification. Covers documentation, validation, training, and continuous improvement.

Automate SOP enforcement, residue tracking, and documentation with CLEEN's all-in-one platform.
Leucine's Batch Execution Software ensures 60% faster documentation and error-free compliance.
Request a Demo
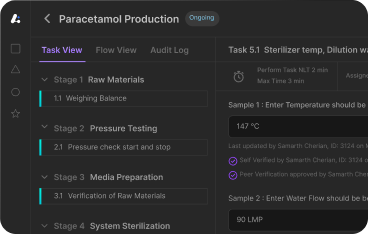
LEUCINE 10X MES
Experience the Future of Pharma
Integrate your entire shop floor in digital environment, uncover powerful insights and increase productivity.

LEUCINE 10X MES
Experience Paperless Production.
Achieve paperless batch record execution, enhance efficiency, accuracy, and collaboration on the shop floor.
FDA TRACKER
Free FDA Form 483 Analytics Tool
Mitigate compliance risks in your pharma processes with our large repository of in-depth insights
Related Resources




















