Batch Manufacturing Software: How Modern Solutions Prevent FDA 483 Observations and Manufacturing Failures

The pharmaceutical industry faces mounting regulatory pressure, with batch production documentation serving as the foundation of manufacturing compliance. Recent FDA Tracker data reveals that documentation-related violations continue among the top FDA 483 observations, making batch manufacturing software essential for pharmaceutical operations.
The Critical Need for Batch Manufacturing Software
Recent FDA 483 Observation Analysis:
- Issue Date: January 8, 2025
- Observation: "The firm does not maintain complete and accurate batch production and control records for its drug products."
- Critical Finding: Missing documentation for API Batch #1123 critical process verification
- Regulatory Impact: Process validation and data integrity compromised
This observation highlights why pharmaceutical companies need robust batch manufacturing software to maintain FDA compliance. Traditional paper-based systems create documentation gaps that modern batch manufacturing software solutions eliminate through automated data capture and integrated workflows.
Based on FDA Tracker analytics, facilities using advanced batch manufacturing software experience 45% fewer batch record violations compared to companies relying on manual documentation processes.
Regulatory Framework: Why Batch Manufacturing Software is Essential
21 CFR Part 211.188 Requirements
Modern batch manufacturing software must address comprehensive FDA requirements under 21 CFR Part 211.188:
- Complete documentation of manufacturing processes
- Identity and measure tracking for all components
- Comprehensive processing, packaging, and quality control records
- Investigation documentation for unexplained discrepancies
- Laboratory test result integration
ALCOA+ Compliance Through Batch Manufacturing Software
Leading batch manufacturing software platforms ensure ALCOA+ data integrity principles:
- Attributable: Electronic signatures identify personnel for each operation
- Legible: Digital documentation supports regulatory review requirements
- Contemporaneous: Real-time data capture during manufacturing activities
- Original: Authentic data recorded at manufacturing points
- Accurate: Automated systems eliminate manual documentation errors
- Complete: Comprehensive records covering all critical process steps
- Consistent: Standardized documentation across all batches
- Enduring: Permanent digital records supporting product lifecycle
- Available: Instant access for regulatory inspections and business decisions
Companies implementing comprehensive batch manufacturing software demonstrate superior regulatory compliance compared to legacy documentation systems.
Business Impact: The Cost of Inadequate Systems
Financial Consequences Without Batch Manufacturing Software
FDA Tracker research quantifies the cost of inadequate batch documentation systems:
Direct Financial Impact:
- Average remediation costs: $3.2 million per facility for batch record violations
- Product holds: 73% of incomplete record violations result in manufacturing delays
- Waste increase: 12% higher batch rejection rates without proper systems
- Investigation expenses: Additional $150,000-$300,000 per cycle
Operational Disruption:
Manufacturing facilities without modern batch manufacturing software face systematic challenges that impact business continuity. Documentation bottlenecks create production delays averaging 8-12 weeks during remediation periods. Customer relationships suffer when 45% of companies report supply interruptions related to compliance issues.
Regulatory Escalation Patterns
Companies lacking proper batch manufacturing software face concerning regulatory trends:
- Warning Letter Risk: 52% of batch record violations escalate within 12 months
- Consent Decree Probability: 23% higher risk for facilities with chronic documentation issues
- Import Restrictions: 38% of repeat violators face trade limitations
- Inspection Frequency: 2.3x more frequent FDA inspections for non-compliant facilities
Learn how electronic batch manufacturing software prevents regulatory escalation
Why Traditional Systems Fail: The Digital Transformation Imperative
Manual Documentation Limitations
Legacy approaches create systematic vulnerabilities that modern batch manufacturing software eliminates:
Process Documentation Gaps:
Traditional paper-based systems cannot capture the real-time manufacturing data that FDA inspectors expect. Manufacturing operators spend excessive time on documentation rather than value-added activities, leading to incomplete records. Critical process parameters go undocumented when manual systems cannot integrate with manufacturing equipment effectively.
System Integration Challenges:
Without integrated batch manufacturing software, companies struggle to demonstrate comprehensive process control. Equipment data, environmental monitoring, and material tracking operate independently, creating information silos that prevent complete batch documentation. This disconnect makes it impossible to verify that documented procedures reflect actual manufacturing conditions.
Resource and Training Inconsistencies:
Manual systems rely heavily on individual operator experience and diligence. During high-volume production periods, documentation quality deteriorates as manufacturing throughput takes priority. Training variations across shifts and sites lead to inconsistent documentation standards that create regulatory vulnerability during FDA inspections.
Modern FDA Expectations
Evolving Regulatory Standards:
FDA inspections increasingly focus on integrated data systems rather than simple documentation compliance. Inspectors expect batch manufacturing software that demonstrates continuous process monitoring and real-time decision-making capabilities. The agency's emphasis on pharmaceutical quality systems requires digital approaches that manual methods cannot deliver.
Manufacturing Complexity Requirements:
Modern pharmaceutical production involves multi-step processes with critical quality attributes requiring continuous monitoring. Traditional approaches cannot capture the detailed verification data that regulatory agencies expect, particularly for API manufacturing where process conditions directly impact product quality and patient safety.
Leucine's Batch Manufacturing Software Solution
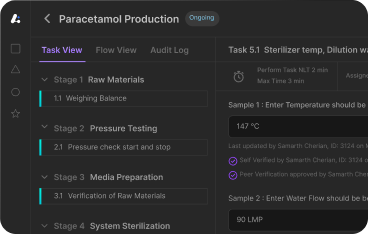
Leucine's Electronic Batch Record (EBR) software represents the next generation of batch manufacturing software, specifically designed to address FDA compliance gaps identified in recent regulatory observations.
Advanced Automation Features
Real-Time Manufacturing Integration:
Leucine's batch manufacturing software automatically captures all production activities contemporaneously, ensuring complete documentation throughout manufacturing cycles. The system integrates directly with process equipment, environmental systems, and material tracking to create comprehensive batch records without manual intervention.
Equipment parameters, process conditions, and operator activities receive automatic documentation with electronic signatures and timestamps. Unlike traditional approaches, every critical manufacturing step includes complete verification documentation that exceeds FDA expectations for process validation adherence.
Comprehensive Process Intelligence:
The platform connects batch execution with quality control, environmental monitoring, and material management through unified batch manufacturing software architecture. Manufacturing deviations trigger automated investigation workflows, ensuring proper exception documentation and resolution tracking within batch records.
Regulatory Compliance Capabilities
21 CFR Part 11 Electronic Signatures:
Leucine's batch manufacturing software provides native electronic signature functionality ensuring every record entry maintains attributability, authentication, and auditability. Manufacturing personnel can review and approve documentation electronically, creating complete audit trails that satisfy FDA data integrity requirements.
Automated Review Workflows:
The system guides quality personnel through systematic batch record review processes, verifying that all required documentation meets completion standards before batch release. Automated verification checks ensure critical process parameters remain within specifications and all required testing receives proper documentation.
Exception Management Integration:
When manufacturing deviations occur, the batch manufacturing software automatically initiates comprehensive documentation workflows capturing investigation details and corrective actions directly within batch records. This integration ensures complete traceability of exception handling, preventing the gaps FDA inspectors consistently identify during facility inspections.
Schedule a demonstration of advanced batch manufacturing software capabilities
Integrated Manufacturing Quality Ecosystem
Seamless System Integration
Leucine's batch manufacturing software integrates with complementary quality management solutions:
Production Logbook Integration:
Equipment maintenance, calibration, and performance data flows automatically into batch records, ensuring complete equipment traceability. Manufacturing personnel access equipment history and performance trends directly within batch execution workflows.
Batch Intelligence Analytics:
Advanced analytics identify patterns and compliance risks before regulatory inspections. The platform provides trending analysis of documentation completeness and identifies improvement opportunities for training and process optimization.
AI Investigator Connectivity:
When batch record discrepancies occur, AI-powered investigation workflows automatically assess cross-batch impacts and generate comprehensive documentation that exceeds FDA expectations for thoroughness and scientific rigor.
Proven Return on Investment
Quantifiable Business Benefits:
Companies implementing Leucine's batch manufacturing software achieve measurable operational improvements: 65% reduction in batch documentation preparation time, allowing manufacturing focus on value-added activities. Documentation accuracy improves 89%, virtually eliminating manual errors that create regulatory compliance risks.
Manufacturing cycle times decrease 23% on average as automated workflows eliminate documentation bottlenecks and review delays. Batch release times improve significantly when quality personnel access complete, verified documentation immediately upon manufacturing completion.
Regulatory Performance Enhancement:
Organizations using Leucine's batch manufacturing software report 78% fewer batch record-related FDA observations during regulatory inspections. The comprehensive documentation and audit trail capabilities provide regulatory confidence that traditional paper systems cannot deliver.
Transform manufacturing operations with proven batch manufacturing software
Conclusion: Digital Transformation for Regulatory Excellence
The pharmaceutical industry requires advanced batch manufacturing software to meet modern regulatory expectations and operational demands. Recent FDA observations demonstrate that traditional documentation approaches create unacceptable compliance risks and business disruption.
FDA Tracker data consistently shows companies implementing comprehensive batch manufacturing software achieve superior regulatory outcomes while optimizing manufacturing efficiency. Modern pharmaceutical operations demand integrated digital platforms ensuring complete documentation, real-time compliance monitoring, and comprehensive audit readiness.
Leucine's batch manufacturing software provides pharmaceutical companies with the advanced capabilities needed to eliminate documentation gaps, exceed FDA expectations, and transform manufacturing operations. The platform's proven performance demonstrates that proactive digital transformation prevents regulatory observations while driving operational excellence.
Protect your manufacturing operations from regulatory risk. Contact Leucine today to discover how our batch manufacturing software strengthens compliance programs and optimizes pharmaceutical operations.
Frequently Asked Questions
Q: What specific features should pharmaceutical companies look for in batch manufacturing software to ensure FDA compliance?
Effective batch manufacturing software must provide 21 CFR Part 11 compliant electronic signatures, real-time data integration with manufacturing equipment, automated ALCOA+ data integrity controls, and comprehensive audit trails. Leucine's batch manufacturing software includes all these essential features plus automated workflow management, exception handling integration, and advanced analytics capabilities. The system ensures complete batch documentation without manual gaps while providing regulatory authorities with immediate access to comprehensive manufacturing records that exceed FDA expectations for data integrity and process validation.
Q: How does batch manufacturing software prevent the regulatory escalations that traditional paper systems create?
Companies using advanced batch manufacturing software experience significantly lower regulatory escalation rates because digital systems eliminate the documentation gaps that trigger FDA enforcement actions. FDA Tracker analysis shows 52% of paper-based batch record violations escalate to Warning Letters, while facilities implementing comprehensive batch manufacturing software report 78% fewer FDA observations. Digital platforms provide complete audit trails, real-time compliance monitoring, and integrated quality management that demonstrate regulatory compliance proactively rather than reactively responding to inspection findings.
Q: What return on investment can pharmaceutical manufacturers expect from implementing modern batch manufacturing software?
Pharmaceutical companies typically achieve 3-5x ROI within 18 months through multiple value streams when implementing Leucine's batch manufacturing software: 65% reduction in documentation time, 89% improvement in accuracy, 23% faster manufacturing cycles, and 78% fewer regulatory observations. For facilities processing 200 batches annually, this translates to $1.8-3.2M in direct operational savings plus significant risk mitigation from avoiding FDA enforcement actions, product holds, and supply chain disruptions that cost $5-15M per incident.
Ready to Transform Your Batch Manufacturing?
See how Leucine's AI-powered batch manufacturing software can eliminate FDA compliance risks and streamline your operations. Join 300+ pharmaceutical facilities already benefiting from our proven solutions.
✓ 65% faster documentation ✓ 78% fewer FDA observations ✓ Real-time compliance monitoring
Book a Free DemoLearn MoreGet a personalized demo tailored to your manufacturing needs. No commitment required.