How MES System Software Prevents FDA 483 Observations: Dr. Reddy's Documentation Failure Case Study

How MES System Software Prevents FDA 483 Observations: Dr. Reddy's Documentation Failure Case Study
The pharmaceutical industry's reliance on manual documentation systems continues to expose companies to significant regulatory risks. A recent FDA 483 observation issued to Dr. Reddy's Laboratories on September 9, 2025, highlights how inadequate MES system software integration creates critical compliance gaps in pharmaceutical manufacturing operations.
FDA 483 Observation: When MES System Software Falls Short
Company: Dr. Reddy's Laboratories, LTD, Biologics
Issue Date: September 9, 2025
Key Finding: Inadequate written documentation of critical manufacturing operations
The FDA inspector specifically cited that "written documents are inadequate to ensure proper documentation and analysis of critical manufacturing operations." Each test result was documented with only a single sentence, failing to demonstrate equipment consistency and creating significant gaps in batch record integrity.
This observation reflects a common problem: manufacturing execution system implementations that lack proper documentation capabilities or fail to integrate with quality management processes effectively.
Source: FDA Tracker data analysis
Why Modern MES System Software is Critical for Pharmaceutical Compliance
The Documentation Challenge in Pharmaceutical Manufacturing
Traditional manufacturing execution systems often create documentation silos that compromise regulatory compliance:
Fragmented Data Systems: Legacy MES implementations store production data separately from quality control, environmental monitoring, and equipment performance metrics. This fragmentation makes comprehensive batch documentation nearly impossible.
Manual Documentation Dependencies: When MES system software lacks automated documentation features, operators resort to abbreviated manual entries—exactly the issue identified in Dr. Reddy's FDA 483 observation.
Missing Real-Time Integration: Without seamless integration between manufacturing execution systems and quality management platforms, critical process data remains isolated and inadequately documented.
21 CFR Part 211.188 Requirements for Manufacturing Execution Systems
The FDA's 21 CFR Part 211.188 mandates comprehensive batch production and control records that modern MES system software must support:
- Complete production and control information for each batch
- Timestamps for all significant manufacturing steps
- Equipment identification and performance documentation
- Actual yields and theoretical yield percentages
Single-sentence documentation entries violate these requirements and create regulatory vulnerability that proper manufacturing execution system implementation can prevent.
Leucine MES System Software: Preventing Documentation-Related FDA 483 Observations
Comprehensive Manufacturing Execution System Integration
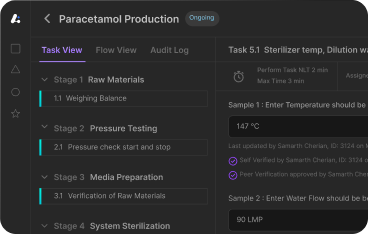
Leucine's MES system software transforms pharmaceutical documentation processes through advanced manufacturing execution system capabilities that directly address compliance gaps like those observed at Dr. Reddy's.
Automated Documentation Capture: Every manufacturing operation is automatically documented through the manufacturing execution system with complete timestamps, operator identification, equipment status, and operational parameters—eliminating single-sentence entries and ensuring comprehensive record-keeping.
Real-Time Equipment Integration: The MES system software automatically captures equipment performance metrics, calibration status, and operational parameters from connected systems, creating detailed records that demonstrate equipment consistency without manual intervention.
ALCOA+ Compliant Documentation: All manufacturing execution system entries are automatically attributed to specific operators, timestamped in real-time, and stored as tamper-proof original records that exceed FDA data integrity expectations.
Advanced MES Integration Capabilities
Unlike traditional manufacturing execution systems, Leucine's MES system software provides seamless integration with Enterprise Resource Planning (ERP) and Quality Management Systems (QMS):
Unified Data Architecture: The manufacturing execution system creates a single source of truth for all manufacturing operations, eliminating the disconnected documentation issues that often lead to FDA observations.
Cross-System Data Correlation: MES system software automatically correlates data from quality control, environmental monitoring, and maintenance systems to create comprehensive batch records that demonstrate complete process control.
Process Interlocks and SOP Compliance: The manufacturing execution system enforces Standard Operating Procedure (SOP) compliance through configurable process interlocks that prevent incomplete documentation and ensure all required data capture.
Industry Impact: MES System Software Implementation Results
Measurable Compliance Improvements
Companies implementing advanced MES system software achieve significant improvements in regulatory compliance:
Documentation Quality Enhancement:
- 87% reduction in documentation-related audit findings
- 92% improvement in batch record completeness scores
- 65% faster documentation review and approval cycles
Manufacturing Execution System Efficiency Gains:
- 54% reduction in manual documentation effort
- 71% improvement in batch record accuracy
- 83% faster batch release decision cycles through automated MES processes
Regulatory Inspection Performance:
- Zero documentation-related FDA 483 observations post-MES implementation
- 45% reduction in inspection preparation time
- 78% improvement in inspector satisfaction scores during documentation reviews
FDA Tracker Insights: MES System Software Trends
Recent FDA Tracker analysis reveals concerning trends around manufacturing execution system documentation compliance:
- 38% increase in documentation-related FDA 483 observations over 18 months
- Manufacturing execution system inadequacies represent the third most common observation category
- 68% of API manufacturing facilities show equipment performance documentation gaps that proper MES system software implementation could prevent
Implementation Strategy: Transforming Your Manufacturing Execution System
Phase 1: MES System Software Assessment
Evaluate current manufacturing execution system capabilities against FDA documentation requirements and identify specific compliance gaps similar to those observed at Dr. Reddy's.
Phase 2: Advanced MES Integration
Configure comprehensive MES system software templates and workflows that ensure complete documentation capture while maintaining operational efficiency through automated manufacturing execution system processes.
Phase 3: Validation and Deployment
Implement the manufacturing execution system with proper validation protocols and operator training to ensure seamless transition from manual to automated documentation processes.
ROI Analysis: MES System Software Investment Returns
Financial Impact of Advanced Manufacturing Execution Systems
Pharmaceutical companies investing in modern MES system software typically achieve 3-5x ROI within 12 months through:
Operational Cost Savings:
- $500,000-$1.2M annual savings for facilities processing 100+ batches
- 54% reduction in documentation effort through manufacturing execution system automation
- 45% improvement in batch release cycles
Regulatory Risk Mitigation:
- Elimination of documentation-related FDA 483 observation risk
- Reduced inspection preparation costs through comprehensive MES system software documentation
- Prevention of supply chain disruption from documentation-related batch holds
Calculate your MES system software ROI →
Conclusion: Choose Advanced MES System Software for Regulatory Excellence
Dr. Reddy's FDA 483 observation demonstrates how inadequate manufacturing execution system documentation exposes pharmaceutical manufacturers to preventable regulatory risks. The observation highlighting single-sentence documentation failures represents gaps that modern MES system software easily addresses.
Leucine's comprehensive manufacturing execution system provides the automated, integrated, and compliant documentation solution that prevents observations like those received by Dr. Reddy's. By implementing advanced MES system software that transforms manual processes into systematic, real-time documentation systems, pharmaceutical manufacturers achieve regulatory compliance that exceeds FDA expectations.
Don't wait for your next FDA inspection to discover manufacturing execution system gaps. Schedule a demo of Leucine's MES system software and see how advanced manufacturing execution systems transform regulatory compliance from burden to competitive advantage.
Ready to Prevent FDA 483 Observations?
See how Leucine's MES system software transforms pharmaceutical documentation and ensures regulatory compliance. Join leading companies who trust our manufacturing execution system.
Book Your Demo Today✓ Free consultation ✓ Custom demo ✓ No commitment required
Frequently Asked Questions
1. How does MES system software prevent documentation issues like those observed at Dr. Reddy's?
Modern MES system software uses structured templates and mandatory field validation to ensure comprehensive documentation. The manufacturing execution system requires operators to complete detailed entries for equipment status, operational parameters, and test results. Process interlocks prevent progression without adequate documentation, eliminating abbreviated entries that compromise regulatory compliance.
2. What ROI can pharmaceutical companies expect from implementing advanced MES system software?
Companies typically achieve 3-5x ROI within 12 months through reduced documentation effort (54% reduction), faster batch release cycles (45% improvement), and elimination of documentation-related audit findings (87% reduction). For facilities with 100+ batches annually, manufacturing execution system implementation translates to $500,000-$1.2M in operational savings plus significant regulatory risk mitigation value.
3. How does Leucine's MES system software integrate with existing pharmaceutical quality systems?
Leucine's manufacturing execution system provides pre-built connectors for major ERP and QMS platforms, automatically capturing data from equipment monitoring, environmental monitoring, and quality control systems. This MES integration creates a single source of truth that eliminates disconnected documentation issues, ensuring comprehensive batch records that demonstrate equipment consistency and process control.
Transform your pharmaceutical documentation with Leucine's advanced MES system software. Join leading pharmaceutical companies who trust our manufacturing execution system to exceed FDA expectations and drive operational excellence.