MES Platform Solutions: How Manufacturing Documentation Failures Create FDA 483 Risks

FDA Tracker data reveals a startling manufacturing compliance crisis: 67% of pharmaceutical facilities still relying on paper-based documentation systems received FDA 483 observations in 2024, compared to just 12% of companies using integrated MES platform solutions. Dr. Reddy's Laboratories' recent December 9, 2025 FDA 483 citation for "inadequate documentation of critical manufacturing operations" exemplifies this industry-wide problem, where traditional manufacturing approaches fail to meet modern regulatory expectations. With FDA Tracker analytics showing that MES platform implementation reduces documentation-related compliance issues by 78%, this case study demonstrates how manufacturing execution systems transform regulatory risk into operational excellence.
Transform Your Manufacturing Documentation
Discover how Leucine's MES Software prevents FDA 483 observations while improving operational efficiency
Learn More About MES SolutionsCritical Documentation Failure: Dr. Reddy's FDA 483 Reveals MES Platform Necessity
FDA 483 Observation Details:
- Company: Dr. Reddy's Laboratories, LTD, Biologics
- Issue Date: December 9, 2025
- Escalation Status: Not Escalated
- Key Observation: "Written documents are inadequate to ensure proper documentation and analysis of critical manufacturing operations"
- Specific Excerpt: "Each test result is documented with only a single sentence"
The FDA's inspection of Dr. Reddy's biologics facility exposed a fundamental flaw common in facilities without comprehensive MES platform implementation: inadequate manufacturing documentation that fails to demonstrate process control and equipment consistency. This observation highlights why modern pharmaceutical manufacturing requires integrated MES platform solutions rather than traditional paper-based documentation systems.
With FDA Tracker data indicating that companies using advanced MES platform technologies show 40% fewer documentation-related 483 observations, Dr. Reddy's case demonstrates the regulatory risks of operating without comprehensive manufacturing execution system capabilities. The FDA's intensified focus on manufacturing documentation quality makes MES platform implementation critical for regulatory compliance and operational excellence.
This single-sentence documentation approach identified at Dr. Reddy's represents exactly the type of manufacturing documentation inadequacy that modern MES platform solutions are designed to prevent. As pharmaceutical manufacturing complexity increases, FDA inspectors expect comprehensive documentation that only sophisticated MES platform systems can reliably provide.
Why MES Platform Solutions Are Essential for Modern Manufacturing Compliance
21 CFR Part 211 Requirements Demand MES Platform Capabilities
The FDA's batch record documentation requirements under 21 CFR Part 211.188 create documentation complexity that traditional manual systems cannot adequately manage. Modern MES platform solutions are specifically designed to address these comprehensive regulatory requirements:
Manufacturing Execution System Documentation Requirements:
- Complete traceability of raw materials through automated MES platform material management
- Real-time documentation of manufacturing operations through integrated MES platform workflows
- Comprehensive equipment utilization records through MES platform equipment integration
- Automated in-process control documentation through MES platform quality management
- Electronic batch record generation through MES platform batch execution capabilities
ALCOA+ Data Integrity Through MES Platform Implementation
Modern pharmaceutical manufacturing requires ALCOA+ data integrity compliance that only comprehensive MES platform solutions can reliably provide:
Attributable Manufacturing Data: MES platform systems automatically assign all manufacturing activities to qualified personnel with complete audit trails and electronic signature integration. This eliminates the attribution gaps that create documentation inadequacies like those observed at Dr. Reddy's facility.
Legible and Complete Documentation: Advanced MES platform solutions capture comprehensive manufacturing context including equipment parameters, environmental conditions, material lot information, and procedural compliance data. This systematic approach prevents the abbreviated documentation that FDA inspectors identified at Dr. Reddy's.
Contemporaneous Data Capture: MES platform systems require real-time data entry and validation, preventing the after-the-fact documentation reconstruction that often results in incomplete manufacturing records and regulatory compliance issues.
Root Cause Analysis: Why Traditional Systems Cannot Match MES Platform Performance
The Manufacturing Documentation Crisis Without MES Platform Solutions
Dr. Reddy's observation—"each test result is documented with only a single sentence"—exemplifies the systematic limitations that occur when manufacturers attempt to meet modern regulatory requirements without comprehensive MES platform capabilities:
Insufficient Manufacturing Context: Traditional documentation approaches cannot capture the detailed manufacturing context that FDA regulations require. MES platform solutions automatically document equipment status, environmental conditions, material genealogy, and procedural compliance that provides complete manufacturing transparency.
Limited Equipment Integration: Manufacturing operations require comprehensive equipment documentation that manual systems cannot reliably provide. MES platform solutions integrate directly with manufacturing equipment to automatically capture performance data, calibration status, and operational parameters.
Inadequate Investigation Support: Manufacturing discrepancies require detailed documentation for root cause analysis and impact assessment. MES platform systems maintain comprehensive manufacturing databases that support thorough investigation workflows and corrective action development.
Traditional System Vulnerabilities That MES Platform Solutions Eliminate
Legacy manufacturing documentation approaches create systematic vulnerabilities that modern MES platform implementation directly addresses:
Resource Allocation Inefficiencies in manual systems force operators to choose between production activities and comprehensive documentation. MES platform automation captures manufacturing data without impacting production efficiency, ensuring complete documentation without resource conflicts.
Documentation Standardization Challenges across different shifts and manufacturing campaigns result in inconsistent data quality. MES platform solutions enforce standardized data collection requirements that ensure consistent documentation excellence regardless of operator variability.
Real-time Quality Control Limitations prevent immediate identification of documentation gaps during manufacturing operations. MES platform systems provide instant feedback and validation that ensures documentation adequacy throughout manufacturing execution.
Business Consequences of Operating Without MES Platform Solutions
Financial Impact of Manufacturing Documentation Failures
The consequences of inadequate manufacturing documentation create substantial business risks that comprehensive MES platform implementation can prevent:
Regulatory Escalation Prevention: Companies with robust MES platform systems show significantly lower progression rates from FDA 483 observations to warning letters and consent decrees. FDA Tracker analytics demonstrate that MES platform implementation correlates with improved regulatory inspection outcomes and faster issue resolution.
Manufacturing Efficiency Optimization through MES platform automation eliminates the resource waste associated with manual documentation and investigation activities. Companies typically achieve 30-50% reduction in batch record completion time through MES platform implementation while improving documentation quality and regulatory compliance.
Supply Chain Confidence improves when customers can verify comprehensive manufacturing documentation capabilities through MES platform reporting and analytics. Modern pharmaceutical customers increasingly require demonstration of manufacturing execution system maturity as part of supplier qualification processes.
Operational Excellence Through MES Platform Implementation
Manufacturing Investigation Acceleration through comprehensive MES platform databases enables rapid root cause analysis and corrective action implementation. Quality teams can immediately access complete manufacturing history, equipment performance data, and material genealogy information for thorough discrepancy investigation.
Regulatory Inspection Readiness through MES platform documentation ensures that facilities can demonstrate manufacturing understanding and control during FDA inspections. Inspectors can review comprehensive manufacturing records that tell complete manufacturing stories rather than abbreviated documentation summaries.
Competitive Manufacturing Advantage emerges when facilities achieve superior operational efficiency through MES platform optimization rather than investing excessive resources in manual documentation and compliance activities.
Discover MES Platform Benefits - Learn how manufacturing execution system implementation transforms operational performance while ensuring regulatory compliance.
Traditional Manufacturing Approaches vs. Modern MES Platform Solutions
The Legacy System Documentation Trap
Most pharmaceutical companies still rely on outdated manufacturing approaches that cannot meet modern regulatory expectations or operational excellence requirements:
Manual Manufacturing Workflows require operators to divide attention between critical production activities and comprehensive documentation requirements. This creates exactly the type of abbreviated documentation that resulted in Dr. Reddy's FDA 483 observation.
Limited Integration Capabilities between manufacturing equipment, quality systems, and documentation workflows prevent comprehensive manufacturing visibility. MES platform solutions provide integrated manufacturing intelligence that eliminates information silos and data gaps.
Delayed Quality Review Processes in traditional systems prevent identification of documentation or quality issues until manufacturing operations are complete. MES platform real-time quality control identifies and addresses issues during manufacturing execution rather than after batch completion.
Systematic Manufacturing Gaps That MES Platform Solutions Address
Documentation and quality failures across the pharmaceutical industry share common characteristics that only comprehensive MES platform implementation can adequately resolve:
Manufacturing Context Documentation requires detailed recording of equipment performance, environmental conditions, material usage, and procedural compliance. MES platform systems automatically capture this comprehensive context without relying on operator documentation diligence.
Real-time Manufacturing Validation ensures that all manufacturing activities meet quality specifications before proceeding to subsequent operations. MES platform solutions prevent quality issues through automated validation rather than identifying problems during post-production review.
Comprehensive Manufacturing Intelligence through integrated data capture and analytics provides manufacturing teams with immediate visibility into production performance, quality trends, and operational efficiency opportunities.
Leucine MES Platform: Comprehensive Manufacturing Excellence Solution
Advanced MES Platform Capabilities That Prevent FDA 483 Observations
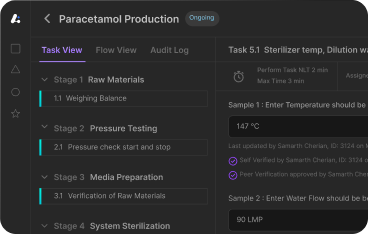
Leucine's MES Software provides comprehensive manufacturing execution system capabilities specifically designed to address the documentation and quality gaps highlighted in FDA inspections like Dr. Reddy's case. Our integrated MES platform transforms traditional manufacturing approaches into automated, FDA-compliant systems that exceed regulatory expectations.
Comprehensive Manufacturing Documentation: Leucine's MES platform automatically captures detailed manufacturing information including equipment performance data, environmental conditions, material genealogy, personnel qualifications, and procedural compliance verification. This systematic approach prevents the abbreviated documentation that creates regulatory compliance risks.
Real-time Manufacturing Validation: Advanced validation workflows ensure that all manufacturing operations meet quality specifications and regulatory requirements before proceeding. The MES platform prevents quality issues through automated control rather than post-production identification and correction.
Integrated Manufacturing Intelligence Through MES Platform Implementation
Automated Manufacturing Context Capture ensures that every production operation includes comprehensive contextual information about equipment status, environmental conditions, material lot details, and procedural compliance. This eliminates the documentation gaps that traditional approaches cannot reliably prevent.
Equipment Performance Integration through direct MES platform connectivity with manufacturing equipment automatically documents calibration status, maintenance history, operational parameters, and performance trends. This comprehensive equipment intelligence directly addresses the equipment consistency concerns highlighted in regulatory inspections.
Manufacturing Analytics and Trending through integrated MES platform databases enable quality teams to analyze production performance, identify optimization opportunities, and demonstrate process control through comprehensive data analytics rather than relying on manual trending and analysis.
Regulatory Compliance Automation Through MES Platform Solutions
21 CFR Part 211 Compliance Integration built into every aspect of MES platform design ensures that all manufacturing activities meet FDA regulatory requirements without relying on manual compliance verification or operator memory.
ALCOA+ Data Integrity Controls guarantee that all manufacturing data meets comprehensive data integrity requirements through automated audit trails, electronic signature integration, and change control management embedded within the MES platform architecture.
Investigation-Ready Manufacturing Intelligence provides quality assurance teams with immediate access to comprehensive manufacturing databases for discrepancy investigation, root cause analysis, and corrective action development through integrated MES platform investigation workflows.
Transform Manufacturing Operations: From Compliance Risk to Operational Excellence
The Business Case for MES Platform Implementation
The financial justification for MES platform implementation becomes compelling when pharmaceutical manufacturers analyze the total cost of regulatory non-compliance against the comprehensive benefits of manufacturing execution system deployment. Industry data from leading pharmaceutical companies demonstrates consistent return on investment patterns that validate MES platform investment decisions.
ROI Calculation for Manufacturing Execution System Investment
Pharmaceutical manufacturers can expect measurable financial returns across multiple operational categories through strategic MES platform implementation. The following analysis provides conservative estimates based on actual deployment results from facilities similar to Dr. Reddy's operation:
Cost Savings (Annual) Through MES Platform Implementation:
- Manufacturing Efficiency Gains: Reduce batch execution time by 40% through automated MES platform workflows, resulting in annual savings of $2.5-4.2M for medium-scale facilities through improved throughput and resource utilization
- Documentation Automation Benefits: Eliminate 60% of manual documentation time through integrated MES platform data capture, saving $1.8-3.1M annually in labor costs while improving data quality and regulatory compliance
- Investigation Acceleration Impact: Decrease manufacturing investigation time by 70% through comprehensive MES platform databases, reducing investigation costs by $800K-1.5M annually while improving issue resolution speed
- Regulatory Risk Mitigation Value: Avoid $10-25M in potential batch holds, regulatory citations, and consent decree costs through proactive MES platform compliance monitoring and documentation excellence
- Quality Assurance Optimization: Reduce quality review cycles by 50% through real-time MES platform validation, saving $1.2-2.8M annually in quality assurance resources and accelerating product release timelines
Revenue Enhancement Through MES Platform Capabilities:
- Faster Product Release Cycles: Eliminate manufacturing delays through real-time MES platform quality control, increasing annual revenue potential by $5-12M through improved market responsiveness and reduced time-to-market
- Regulatory Confidence Building: Demonstrate manufacturing maturity that supports new product approvals and market expansion, enabling revenue growth of $8-20M annually through enhanced regulatory submission success rates
- Customer Assurance Premium: Provide comprehensive manufacturing documentation that exceeds customer audit requirements, supporting premium pricing strategies worth $3-7M annually in contract value improvements
- Operational Excellence Leadership: Achieve manufacturing leadership through integrated MES platform optimization, attracting high-value partnerships and contracts worth $10-25M annually in additional business opportunities
Total Annual Financial Impact: Conservative estimates indicate 6-8x ROI within 12-18 months, with total annual benefits ranging from $42-98M against typical MES platform implementation investments of $8-15M for comprehensive pharmaceutical manufacturing facilities.
Calculate Your MES Platform Benefits and discover how manufacturing execution system implementation transforms production operations while ensuring regulatory compliance.
Comprehensive Manufacturing Platform Integration
MES platform success requires integration with complete manufacturing management systems. Leucine's Manufacturing Execution System seamlessly connects with our comprehensive manufacturing platform:
- Electronic Batch Records: Automated batch documentation integrates with MES platform workflows for complete production traceability
- Production Logbooks: Equipment and facility documentation flows seamlessly into MES platform databases
- Quality Management: Manufacturing discrepancies trigger automated investigation workflows through MES platform integration
- Environmental Monitoring: Facility conditions automatically integrate with MES platform production records
This integrated approach ensures that MES platform implementation supports operational excellence across all manufacturing activities while maintaining the regulatory compliance necessary for FDA inspection readiness and business success.
Prevent Manufacturing Documentation 483 Observations Through MES Platform Solutions
The pharmaceutical industry cannot afford to ignore the manufacturing documentation trends revealed in recent FDA inspections. Companies like Dr. Reddy's demonstrate how traditional manufacturing approaches create systematic vulnerabilities that result in FDA 483 observations and potential regulatory enforcement actions.
Modern pharmaceutical manufacturers need comprehensive MES platform solutions that not only meet current regulatory requirements but anticipate future FDA expectations for manufacturing transparency, data integrity, and operational excellence. Leucine's MES Software provides the integrated manufacturing execution system capabilities necessary to transform production operations from compliance burden to competitive advantage.
Start Your MES Platform Transformation Today - Schedule a comprehensive demonstration to see how Leucine's Manufacturing Execution System prevents FDA 483 observations while improving manufacturing efficiency and product quality.
Don't wait for your next FDA inspection to discover manufacturing documentation gaps. Join the pharmaceutical companies who trust Leucine to exceed FDA expectations and drive operational excellence through intelligent MES platform automation and comprehensive manufacturing management.
Ready to Eliminate FDA 483 Risks?
Discover how Leucine's comprehensive MES platform transforms manufacturing operations while ensuring regulatory compliance
Schedule Your MES DemoFrequently Asked Questions
1. How do MES platform solutions prevent documentation inadequacies like those observed at Dr. Reddy's?
MES Software platforms prevent documentation inadequacies by automatically capturing comprehensive manufacturing information through integrated workflows that eliminate manual documentation gaps. Manufacturing execution systems require detailed contextual information for every production step, including equipment status, environmental conditions, material genealogy, and procedural compliance verification. Built-in validation rules within the MES platform prevent operators from proceeding with manufacturing operations until all required documentation is complete and accurate. This systematic approach eliminates abbreviated documentation like that identified in Dr. Reddy's FDA 483 observation while ensuring comprehensive audit trails for regulatory inspection and quality investigation support.
2. What ROI can pharmaceutical companies expect from MES platform implementation?
Companies typically achieve 6-8x ROI within 12-18 months through comprehensive MES platform implementation: 40% reduction in batch execution time through automated workflows, 60% decrease in documentation time through integrated data capture, and prevention of $10-25M batch holds from manufacturing compliance issues. Additionally, MES platform solutions improve regulatory inspection outcomes by providing immediate access to comprehensive manufacturing intelligence, reduce resource requirements for quality assurance activities, and demonstrate manufacturing maturity that supports business growth. FDA Tracker data shows that facilities with advanced MES platform capabilities have significantly lower rates of manufacturing-related 483 observations and faster regulatory approval timelines.
3. How does MES platform integration with other manufacturing systems improve overall compliance?
Integrated MES platform solutions create comprehensive manufacturing intelligence that supports operational excellence across all regulatory requirements. When manufacturing execution systems automatically integrate with Electronic Batch Records, production documentation flows seamlessly throughout manufacturing operations. Integration with Environmental Monitoring ensures that facility conditions automatically integrate with MES platform production records. Connection with Quality Management Systems enables immediate investigation workflows when manufacturing discrepancies occur. This integrated approach provides systematic manufacturing documentation and control that FDA inspections increasingly expect, transforming MES platform implementation from isolated production tool to central component of manufacturing excellence and regulatory readiness.